Bob Ferguson wrote in Food Safety magazine that there has been substantial growth in the development and use of rapid methods since their introduction in the past 25 years. When these methods were first introduced, they were initially intended for use by food processors in their in-plant laboratories. Over the past decade, with the increase in outsourcing due to concerns for cross-contamination from pathogen samples, fewer and fewer food companies have in-house microbiology laboratories. Most companies are saying that they no longer have a microbiology lab, or, if they do, it does not have capabilities for pathogen analysis since these tests are outsourced. Other outsourcing drivers include client requirements, the need for validation, and the requirement for ISO 17025 accreditation. In general, most of the companies in our survey indicated that tests with a turnaround time of less than 24 hours would be “useful,” and tests with an 8 hour or less turnaround time would be “nice to have.” Very few respondents indicated that a faster test would be advantageous. An analytical test with, for example, results available in 4 hours does not help much if no one can receive and report those results repeatedly. In plant testing will focus on environmental testing. These tests will need to be fast and easy to use. The RMM tests will need to have proven credibility and accuracy. @ https://www.food-safety.com/articles/9398-rapid-testing-methodsthe-future
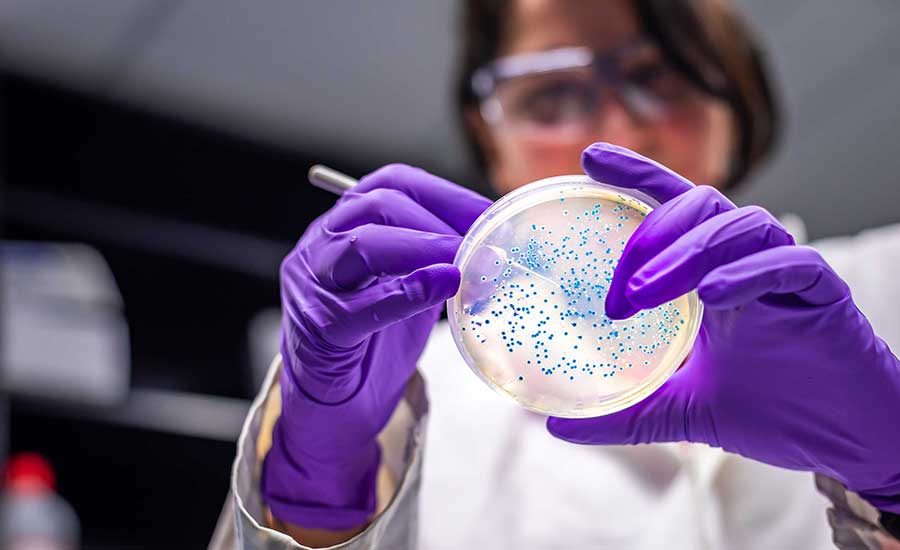
Rapid Microbiology reported that NEMIS Technologies has developed an innovative N-Light™ test in food safety through a unique combination of phages and AquaSpark™ technology. NEMIS revolutionizes pathogen detection by targeting specific bacteria with bacteriophage cocktails, and their method has high sensitivity due to a chemiluminescent reaction. The assay starts with a selective enrichment broth with phages. In the NEMIS N-Light™ tests, bacteriophages play a crucial role by selectively targeting and destroying bacteria competing with the target pathogen. A unique cocktail of bacteriophages targets specific strains that traditional selective agents can’t eliminate. Thus, potential false positives or false negatives are avoided. The assay can detect Listeria monocytogenes and Salmonella. The heart of the technology lies in AquaSpark™. This technology operates through a two-part system. The presence of the target enzyme triggers a reaction with the AquaSpark™ molecule. A reacted molecule rearranges and produces a bright light, which is then detected by the system’s device. The Biosafety Cap ensures the containment of enriched harmful pathogens. NEMIS Technologies’ N-Light™ tests offer a safe, selective, and highly sensitive solution. Ensuring the highest performance standards for L. monocytogenes, Salmonella Risk, E. coli, and ATP detection. @ https://www.rapidmicrobiology.com/news/revolutionizing-pathogen-detection-nemis-technologies-groundbreaking-approach
NEMIS Technologies N-Light™ tests offer a safe, selective, and highly sensitive solution for pathogen detection. This three-pronged approach ensures the h
ruth
Food Safety Magazine reported that the EU, to align with Codex Alimentarius standards, has proposed amendments to its regulations for Listeria monocytogenes in RTE foods. If adopted, the new regulations would go into effect after January 1, 2026. The draft regulations would amend Regulation (EC) No. 2073/2005. At present, the Regulation states that, except for foods intended for infants and special medical purposes, L. monocytogenes cannot be detected in 25 g of RTE foods (that can support pathogen growth over time to exceed 100 cfu/g eventually) before such products leave the production facility. However, Regulation (EC) No. 2073/2005 does not provide that the same criterion applies to these foods once they have left the immediate control of the producing food business operator. The EC is proposing amendments to Annex I of Regulation (EC) No. 2073/2005 to guarantee the same level of public health protection from production to distribution for RTE foods (other than those intended for infants and for special medical purposes) that can support the growth of L. monocytogenes. The proposed amendments would apply the criterion “L. monocytogenes not detected in 25 g” to all situations where covered RTE foods are placed on the market during their shelf-life and for which the producing food business operator has not been able to demonstrate, to the satisfaction of the competent authority, that the level of L. monocytogenes will not exceed the limit of 100 cfu/g throughout the food’s shelf life. @ https://www.food-safety.com/articles/9394-proposed-amendments-would-expand-eu-regulations-on-allowable-levels-of-listeria-in-rte-foods
ruth
Consumer Reports (CR) reported to the USDA that Lunchables contain a troubling high level of lead and sodium. CR tested 12 store-bought Lunchables products from Kraft Heinz and compared them to similar lunch and snack kits from other manufacturers. Although none of the kits exceeded any legal or regulatory limit, the tests uncovered relatively high levels of lead, cadmium, and sodium. CR found that the sodium levels in the store-bought kits ranged from 460 to 740 milligrams per serving, close to ¼ of the daily allowance. Lunchables Extra Cheesy Pizza contains harmful phthalates prevalente in plastic that can be linked to reproductive issues, diabetes, and some cancers. CR concluded that “Lunchables are not a healthy option for kids and shouldn’t be allowed on the menu as part of the National School Lunch Program.” @ https://www.usatoday.com/story/money/food/2024/04/10/lunchables-lead-sodium-consumer-reports-usda/73272198007/
Lunchables contain a high level of lead and sodium, Consumer Reported warned as it petitioned the USDA to remove the products from school lunch menus.