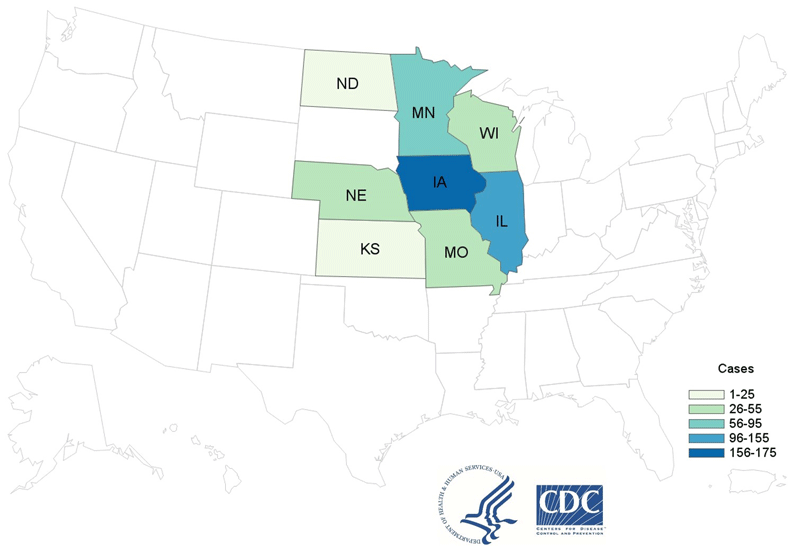
The CDC, along with FDA and state and local partners continue to investigate a multistate outbreak of Cyclospora infections linked to salad products that were made by Fresh Express containing iceberg lettuce, red cabbage, and carrots and that was sold in several regions of the United States. The investigation includes Fresh Express-branded products as well as products made by Fresh Express for retail store brands sold at ALDI, Giant Eagle, Hy-Vee, Jewel-Osco, ShopRite, and Walmart. On June 27, 2020, Fresh Express recalled products containing iceberg lettuce, red cabbage, or carrots. The products are branded with the Fresh Express label or store brand labels of ALDI Little Salad Bar, Giant Eagle, Hy-Vee, Jewel-Osco Signature Farms, ShopRite Wholesome Pantry, and Walmart Marketside. As of July 8, 2020, a total of 509 people with laboratory-confirmed Cyclospora infections associated with this outbreak have been reported from 8 states: Illinois (151), Iowa (160), Kansas (5), Minnesota (63), Missouri (46) Nebraska (48), North Dakota (6), and Wisconsin (30). In Canada, PHAC also issued a notice about the ongoing Cyclospora investigations of the outbreak of Cyclospora infections occurring in three Canadian provinces. Exposure to certain Fresh Express brand salad products containing iceberg lettuce, carrots, and red cabbage, has been identified as a likely source of the outbreak.@ https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2020/index.html
ruth
CDC and federal, state, and local public health partners are investigating an increase in reported cases of Cyclospora infection (cyclosporiasis). Reports of cases tend to increase during summer months in the United States.
ruth
A study by Professor Juan José Quereda Torres, in collaboration with researchers from the Pasteur Institute of Paris published in the Journal of Infectious Diseases, reveals the mechanism of Listeria invasion of human cells. The researchers inactivated the expression of the 165 most essential genes of the host mammal for the infection by Listeria monocytogenes, and have identified for the first time the factors of the host that modulate the rupture of the vacuole and the cytoplasmic access to epithelial cells. Professor Torres research could make it possible to develop new therapies to treat listeriosis in humans and animals in the future. @ https://ruvid.org/ri-world/new-findings-on-infections-caused-by-listeria/
Professor Juan José Quereda Torres has collaborated with the Pasteur Institute of Paris to reveal new findings on how the listeria bacterium invades human and animal cells.
Mary-M
The J. M. Smucker Company today announced a voluntary recall of one lot of Natural Balance® Ultra Premium Chicken & Liver Paté Formula canned cat food due to health concerns likely associated with elevated levels of choline chloride. If pet parents have any product matching the following description in their possession, they should stop feeding it to their cats and dispose of the product. These products are most commonly sold in pet specialty retailers and online throughout the US and Canada. No other Natural Balance® products are impacted by this recall. The Company has received reports of adverse reactions. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/j-m-smucker-company-issues-voluntary-recall-one-lot-natural-balancer-ultra-premium-chicken-liver?utm_campaign=The%20J.%20M.%20Smucker%20Company%20Issues%20Voluntary%20Recall%20of%20One%20Lot%20of%20Natural%20Balance&utm_medium=email&utm_source=Eloqua
The J. M. Smucker Company today announced a voluntary recall of one lot of Natural Balance® Ultra Premium Chicken & Liver Paté Formula canned cat food due to health concerns likely associated with elevated levels of choline chloride.
ruth
Sandra Eskin (The Pew Charitable Trusts) wrote an opinion article stating that reducing the risk on E. coli in romaine lettuce require a coordinated action from farmers, ranchers and government and no single entity can achieve it alone. FDA concluded that the recent outbreaks of E. coli in lettuce came from cattle that grazed in fields near the romaine lettuce. The FDA findings make clear that growers, ranchers, and local, state, and federal agencies must work together to prevent contamination of leafy greens by pathogens commonly present in animal fecal matter. This food safety problem cannot be solved by a single industry or regulatory authority. Allowing cattle to graze near fields that grow romaine lettuce or other leafy greens creates an unacceptable risk to consumers. To solve the problem, produce farmers and cattle ranchers should be at the table, along with federal, state, and local authorities. FDA should lead an effort to find comprehensive solutions to the public health problems created when cattle and produce farms operate in proximity. Without such solutions, Americans may see still more outbreaks linked to romaine lettuce and other leafy greens in the years ahead. @ https://www.pewtrusts.org/en/research-and-analysis/articles/2020/07/02/fda-says-cattle-likely-source-of-e-coli-that-contaminated-romaine-in-2019
Dangerous E. coli bacteria that caused three foodborne illness outbreaks in late 2019 most likely came from cattle that grazed near fields of romaine lettuce or leafy greens, according to a recent U.S. Food and Drug Administration report.