Health Canada recalled multiple brands of frozen mango because they may be contaminated with Hepatitis A. The recalled brands of mangos are Nature’s Touch frozen mango, Compliments mango mania, Irresistibles frozen mango chunks, and President’s Choice frozen mango chunks. The agency reported that it had received information about people becoming sick after eating the recalled products. Health Canada reported that two people in Quebec, 1 in N.S., tested positive for Hepatitis A after consuming the frozen mangoes. The frozen mangoes were distributed in Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Possibly National, Prince Edward Island, Quebec, and Saskatchewan. No hospitalizations or deaths have been reported. @ https://inspection.canada.ca/food-recall-warnings-and-allergy-alerts/2021-07-30/eng/1627691106085/1627691112044
Nature’s Touch Frozen Food Inc. is recalling various frozen mangoes from the marketplace due to possible Hepatitis A contamination.
ruth
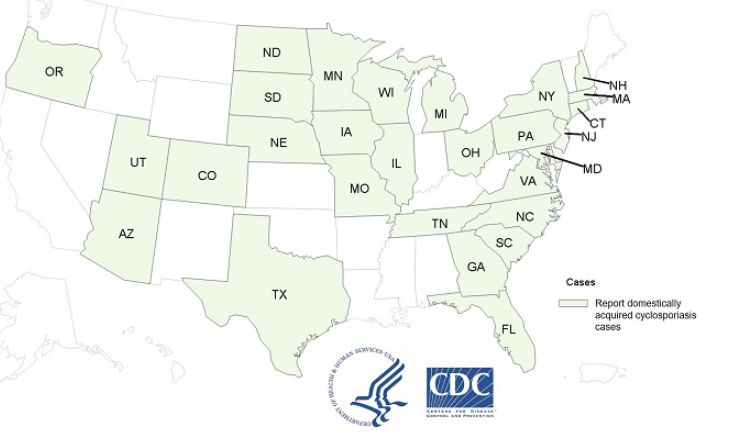
The CDC reported that it could not find the common source for this year’s outbreak of Cyclosporiasis. The number of reported cases of domestically acquired Cyclosporiasis illnesses has increased by 254 to 462 laboratory-confirmed cases since the last update on July 14, 2021. Cases continue to be reported to CDC. Illness onset has been reported to CDC by 29 jurisdictions, including 28 states and New York City. At least 41 people have been hospitalized; no deaths have been reported. @ https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2021/seasonal/index.html#states
CDC and federal, state, and local public health partners are investigating an increase in reported cases of Cyclospora infection (cyclosporiasis). Reports of cases tend to increase during summer months in the United States.
ruth
According to the FDA, BrightFarms extended its recall of salad greens to include BrightFarms branded Fresh Baby Spinach from the American marketplace due to suspected Salmonella contamination. The recall expansion included packaged salad greens past the expiration date. It was produced in its Rochelle, Illinois (Ogle County) greenhouse farm sold in Illinois, Wisconsin, Iowa, Indiana, and Michigan due to potential contamination Salmonella. The recalled products were packaged in clear, plastic clamshells with “best by” dates through 7/26/2021. The products were sold by retailers listed in the July 15 recall notice. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/brightfarms-announces-voluntary-recall-expansion-packaged-salad-greens-sold-illinois-wisconsin-iowa
BrightFarms today initiated a voluntary recall expansion of additional packaged salad greens that are past expiration date and were produced in its Rochelle, Illinois (Ogle County) greenhouse farm sold in Illinois, Wisconsin, Iowa, Indiana and Michigan due to potential contamination with Salmonella.
ruth
The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced that Greater Omaha Packing (Omaha, Nebraska) recalled approximately 295,236 pounds of raw beef products intended for non-intact use that may be contaminated with E. coli O157:H7. The raw beef products intended for non-intact use were produced on July 13, 2021. These items were distributed to further processors in Illinois, Indiana, Minnesota, and Nebraska. The problem was discovered when FSIS collected a routine product sample that confirmed positive for the presence of E. coli O157:H7. There have been no confirmed reports of adverse reactions due to the consumption of these products. @ https://www.fsis.usda.gov/recalls-alerts/greater-omaha-packing-recalls-raw-beef-products-due-possible-e.-coli-o157h7