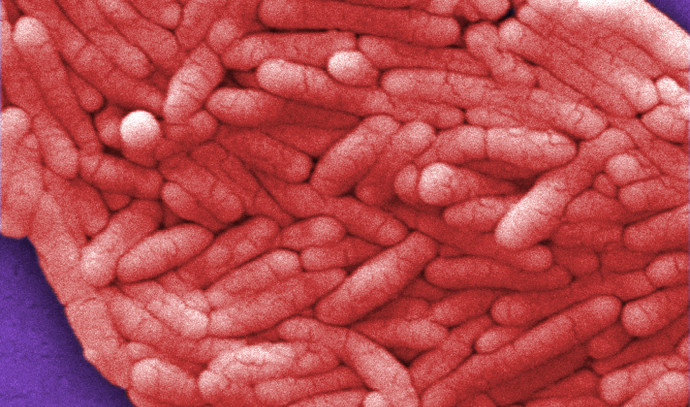
The European Centre for Disease Prevention and Control reported that since August 2022 and as of 12 July 2023, 92 cases of Salmonella Senftenberg have been reported in Austria (5), Belgium (4), Czechia (4), Estonia (1), Finland (12), France (16), Germany (26), Ireland (1), the Netherlands (5), Norway (1), Sweden (11), the United Kingdom (4), and the United States (2). One patient has died of the infection. The first case was reported in France with an isolation date of 22 August 2022, and the most recent case was reported on 24 June 2023 in Sweden. Most cases were reported between October 2022 and March 2023, with a decline in the number of countries with exposures after December. In Austria, Germany, and France, cherry-like tomatoes were identified as the food exposure most reported by interviewed cases. The outbreak strain was detected in France from a mixed salad dish containing cherry tomatoes and green leafy vegetables, prepared on 17 August 2022 but not served. Tomatoes from the salad in France and tomatoes in Austria were suspected as the vehicle of infections by national authorities and were traced back to wholesalers in Germany, the Netherlands, and Spain and to growers in the Netherlands, Spain, and Morocco. In the absence of microbiological evidence from the tomatoes, the source of the infections could not be established. The genetic similarity of the human outbreak strains suggests a likely common source(s) causing a prolonged, cross-border foodborne outbreak with cases intermittently reported in 11 EU/EEA countries, the UK, and the US for about 10 months. The contamination may have originated from farms growing tomatoes. @ https://www.ecdc.europa.eu/en/publications-data/multi-country-outbreak-salmonella-senftenberg-st14-infections