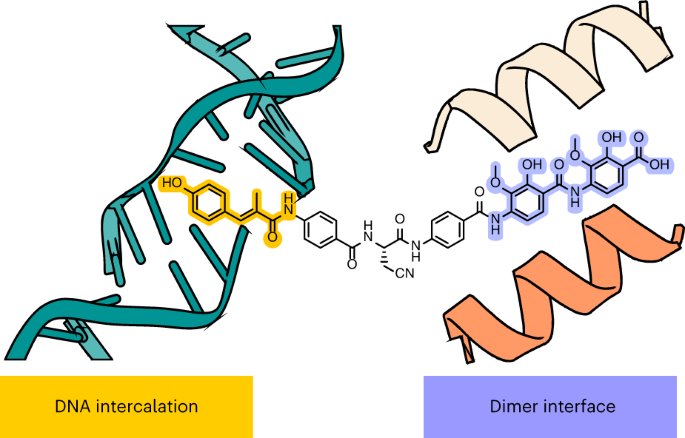
A new publication describes a novel antibiotic, Albicidin, with bactericidal activity towards fluoroquinolone-resistant Gram-negative pathogens (Michalczyk, E., Hommernick, K., Behroz, I. et al. Molecular mechanism of topoisomerase poisoning by the peptide antibiotic Albicidin. Nat Catal 6, 52–67 (2023)). Albicidin employs a dual binding mechanism where one end of the molecule obstructs the crucial gyrase dimer interface while the other intercalates between the fragments of cleaved DNA substrate. Therefore, Albicidin efficiently locks DNA gyrase, preventing it from religating DNA and completing its catalytic cycle. Two additional structures of this trapped state were determined using synthetic Albicidin analogues, demonstrating improved solubility and activity against various gyrase variants and E. coli topoisomerase IV. The extraordinary promiscuity of the DNA-intercalating region of Albicidin and their excellent performance against fluoroquinolone-resistant bacteria holds great promise for the development of last-resort antibiotics.@ https://doi.org/10.1038/s41929-022-00904-1
New peptide antibiotic Albicidin with bactericidal activity towards fluoroquinolone-resistant Gram-negative pathogens
Molecular mechanism of topoisomerase poisoning by the peptide antibiotic albicidin - Nature Catalysis
Albicidin is a peptide antibiotic that has shown great promise for inhibiting DNA topoisomerase of fluoroquinolone-resistant Gram-negative pathogens, but its mode of action is not fully clear. Now, cryoelectron microscopy structures of albicidinâgyrase complexes provide detailed insights into the mechanism of this natural product.
No comments