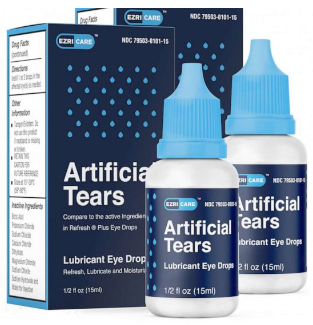
The FDA reported that Global Pharma Healthcare recalled all lots within expiry of their Artificial Tears Lubricant Eye Drops, distributed by /EzriCare, LLC- and Delsam Pharma, due to possible contamination with Pseudomonas aeruginosa. The CDC alerted the FDA to an investigation of a multi-state cluster of Verona Integron-mediated Metallo-β-lactamase (VIM)- and Guiana-Extended Spectrum-β-Lactamase (GES)- producing carbapenem-resistant Pseudomonas aeruginosa (VIM-GES-CRPA) infections might be associated with the use of the artificial tears manufactured by Global Pharma Healthcare. To date, there are 55 reports of adverse events, including eye infections, permanent loss of vision, and a death from a bloodstream infection. The infections, including some found in blood, urine, and lungs, were linked to EzriCare Artificial Tears. Many said they had used the product. The product was distributed Nationwide in the USA over the Internet. EzriCare, the company that markets the eye drops in the U.S., said it has stopped distributing the eye drops. It also has a notice on its website urging consumers to stop using the product. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/global-pharma-healthcare-issues-voluntary-nationwide-recall-artificial-tears-lubricant-eye-drops-due?utm_medium=email&utm_source=govdelivery