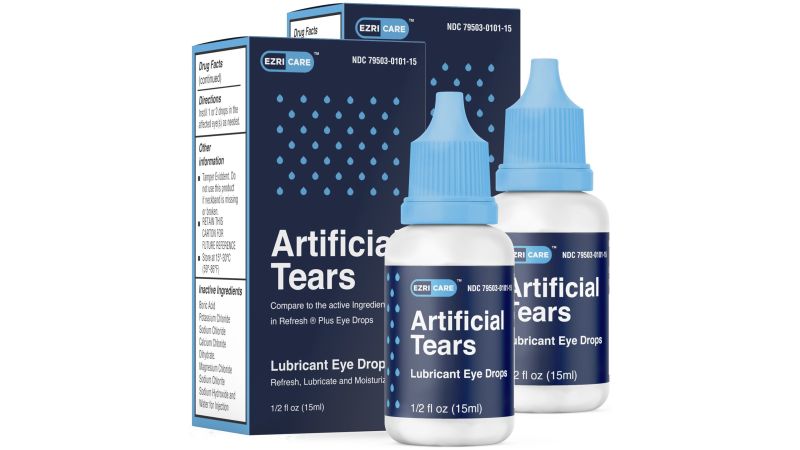
CNN reported that according to an FDA inspection report EzriCare, the manufacturer of Artificial Tears eye drops linked to an outbreak, did not follow proper protocol to prevent contamination of its products. The FDA inspected the Global Pharma Healthcare facility, the manufacturer of EzriCare, in India for 11 days. The inspection resulted in multiple observations by the FDA, including the “manufacturing process that lacked assurance of product sterility,” specifically for batches of products manufactured between December 2020 and April 2022 and shipped to the US. The FDA inspectors also noticed dirty equipment, walls, ceilings, and floors were not smooth and difficult to clean and sterilize. The protocols for cleaning and maintaining equipment were deficient. @ https://www.cnn.com/2023/04/03/health/eye-drops-bacteria-fda-inspection/index.html