An article published in PNAS (December 2019) unveils the resistance mechanism in Enterococcus faecalis. The research has identified a protein (LiaX) that is released by the bacteria cells when it senses antimicrobial molecules. LiaX regulates changes in CM phospholipid architecture, to prevent the drug from destroying it. Targeting this response in multidrug-resistant organisms may be a therapeutic intervention to restore susceptibility to cell envelope-targeting antibiotics and increase the ability of the immune system to clear pathogens. LiaX is the master modulator of resistance. It tells the bacteria to remodel their protective cell envelope, causing Daptomycin (a naturally-occurring lipopeptide antibiotic that kills susceptible gram positive bacteria) to bind away from the septum and allowing the cell to survive. @ https://www.pnas.org/content/early/2019/12/05/1916037116
Antibiotic resistance and virulence mechanism in Enterococcus faecalis unveiled
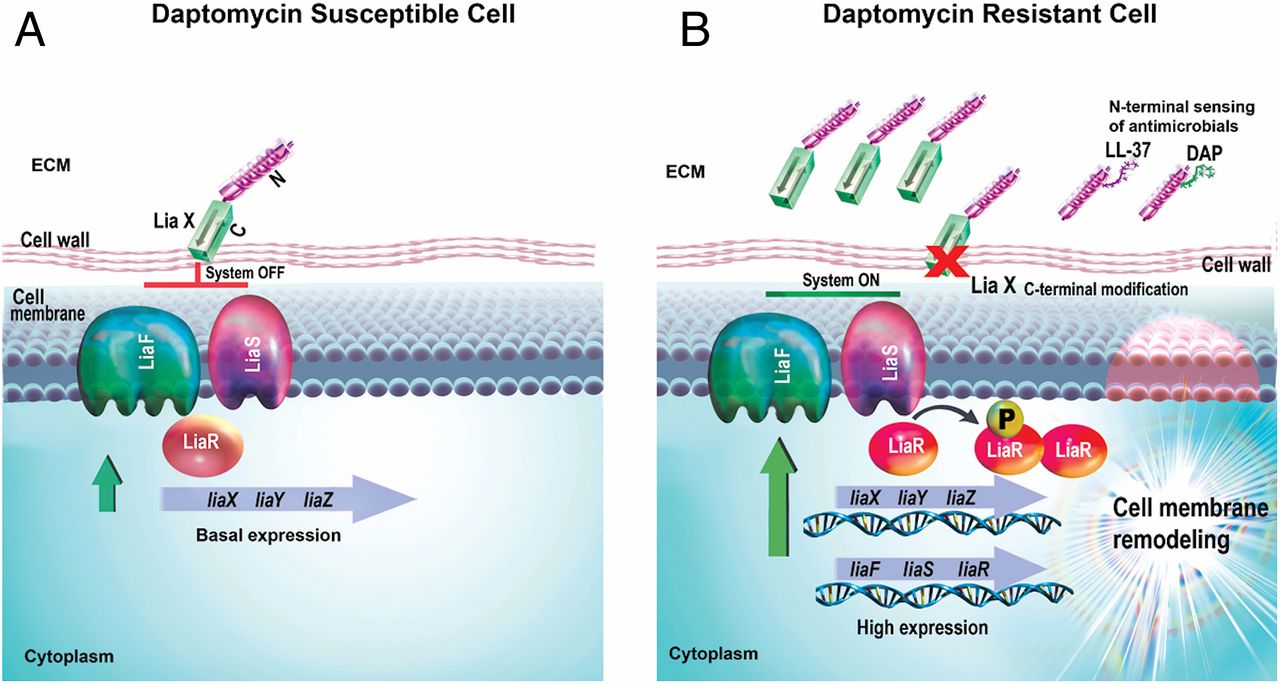
Vancomycin-resistant enterococci are hospital-associated pathogens that evolved resistance to most antibiotics used in clinical practice. Daptomycin, a lipopeptide antibiotic used as frontline therapy for severe multidrug-resistant enterococcal infections, targets the bacterial cell membrane (CM). The LiaFSR stress response system orchestrates daptomycin and antimicrobial peptide (AMP) resistance by modulating CM phospholipid content or localization. Here, we identify a single protein (LiaX) that senses antimicrobial molecules and regulates changes in CM phospholipid architecture. We show that LiaX-mediated modulation of antibiotic and AMP resistance affects virulence during infection caused by a recalcitrant hospital pathogen. Targeting this response in multidrug-resistant organisms may be a therapeutic intervention to restore susceptibility to cell envelope-targeting antibiotics and increase the ability of the immune system to clear pathogens.
Bacteria have developed several evolutionary strategies to protect their cell membranes (CMs) from the attack of antibiotics and antimicrobial peptides (AMPs) produced by the innate immune system, including remodeling of phospholipid content and localization. Multidrug-resistant Enterococcus faecalis, an opportunistic human pathogen, evolves resistance to the lipopeptide daptomycin and AMPs by diverting the antibiotic away from critical septal targets using CM anionic phospholipid redistribution. The LiaFSR stress response system regulates this CM remodeling via the LiaR response regulator by a previously unknown mechanism. Here, we characterize a LiaR-regulated protein, LiaX, that senses daptomycin or AMPs and triggers protective CM remodeling. LiaX is surface exposed, and in daptomycin-resistant clinical strains, both LiaX and the N-terminal domain alone are released into the extracellular milieu. The N-terminal domain of LiaX binds daptomycin and AMPs (such as human LL-37) and functions as an extracellular sentinel that activates the cell envelope stress response. The C-terminal domain of LiaX plays a role in inhibiting the LiaFSR system, and when this domain is absent, it leads to activation of anionic phospholipid redistribution. Strains that exhibit LiaX-mediated CM remodeling and AMP resistance show enhanced virulence in the Caenorhabditis elegans model, an effect that is abolished in animals lacking an innate immune pathway crucial for producing AMPs. In conclusion, we report a mechanism of antibiotic and AMP resistance that couples bacterial stress sensing to major changes in CM architecture, ultimately also affecting host–pathogen interactions.