The Wisconsin Department of Health Services is warning that Del Monte vegetable trays could pose salmonella threat since three people have gotten sick in Wisconsin, one in Minnesota. All sick people reported consuming a Del Monte vegetable tray purchased from a Wisconsin or Minnesota Kwik Trip location prior to their illness. The people reported becoming ill between April 13 and April 27, 2019. The Del Monte vegetable trays associated with the investigation contain broccoli, cauliflower, carrots, and dill dip. The Del Monte vegetable trays may also have been distributed to other retailers in Wisconsin. @ https://www.wisn.com/article/salmonella-linked-to-certain-del-monte-vegetable-trays-bought-at-kwik-trip/27547691
ruth
The Wisconsin Department of Health Services is warning that Del Monte vegetable trays could pose a salmonella threat.
ruth
The CDC is investigating an outbreak related to raw oysters. The FDA is investigating a subset of the outbreak. Between the harvesting dates of February 12, 2019, and April 9, 2019, five ill patients reported eating raw oysters shortly before becoming ill with Shigella flexneri. One of the five patients was hospitalized; there have been no deaths. Laboratory analyses confirmed that reported clinical illnesses matched pathogens found in product samples and traceback information indicated the implicated shellfish were harvested from Estero El Cardon, in Baja California Sur, Mexico. The oysters harvested in Estero El Cardon, Baja California Sur, Mexico, were distributed to California, Nevada, New York, and Arizona with illnesses reported in California, Nevada, and New Hampshire. The New Hampshire case reported eating at a restaurant in California shortly before becoming ill. On May 7, 2019, the Mexican Shellfish Sanitation Program authorities voluntarily closed the growing area of Estero El Cardon and halted oyster harvesting in response to the reported illnesses. @ https://www.fda.gov/food/alerts-advisories-safety-information/fda-investigates-shigella-illnesses-linked-imported-raw-oysters?utm_campaign=Alert%20Shigella%20in%20Raw%20Oysters%2005212019&utm_medium=email&utm_source=Eloqua
Consumers should not purchase oysters marketed as being harvested from Estero El Cardon, in Baja California Sur, Mexico from restaurants.
ruth
Escherichia coli, including Shiga-toxigenic E. coli (STEC), is a serious risk of contamination of fresh produce. Experimental evidence shows that STEC can colonize plants, but this capacity varies by plant. Therefore, an understanding of the impact of various factors on the ability of STEC to grow and establish itself on the plants is required for food safety considerations and risk assessment. Scientists from the University of Aberdeen investigated the ability of STEC to grow and form biofilm in plant extracts, and its relationship to colonization. The bacterial growth rate in plant extracts varied in a plant-dependent and isolate-dependent manner, with spinach leaf lysates supporting the highest rates of growth. Spinach extracts also supported the highest levels of biofilm formation. The highest level of colonization occurred on alfalfa sprouts, though internalization was ten times more prevalent in the leafy vegetables than in sprouted seeds. Overall, the capacities of E. coli to colonize, grow, and internalized within plants or plant-derived matrices was influenced by the isolate type, plant species, plant tissue type, and temperature. @ https://aem.asm.org/content/85/11/e00123-19
Contamination of fresh produce with pathogenic Escherichia coli, including Shiga-toxigenic E. coli (STEC), represents a serious risk to human health. Colonization is governed by multiple bacterial and plant factors that can impact the probability and suitability of bacterial growth. Thus, we aimed to determine whether the growth potential of STEC for plants associated with foodborne outbreaks (two leafy vegetables and two sprouted seed species) is predictive of the colonization of living plants, as assessed from growth kinetics and biofilm formation in plant extracts. The fitness of STEC isolates was compared to that of environmental E. coli isolates at temperatures relevant to plant growth. Growth kinetics in plant extracts varied in a plant-dependent and isolate-dependent manner for all isolates, with spinach leaf lysates supporting the highest rates of growth. Spinach extracts also supported the highest levels of biofilm formation. Saccharides were identified to be the major driver of bacterial growth, although no single metabolite could be correlated with growth kinetics. The highest level of in planta colonization occurred on alfalfa sprouts, though internalization was 10 times more prevalent in the leafy vegetables than in sprouted seeds. Marked differences in in planta growth meant that the growth potential of STEC could be inferred only for sprouted seeds. In contrast, biofilm formation in extracts related to spinach colonization. Overall, the capacity of E. coli to colonize, grow, and be internalized within plants or plant-derived matrices was influenced by the isolate type, plant species, plant tissue type, and temperature, complicating any straightforward relationship between in vitro and in planta behaviors.
IMPORTANCE Fresh produce is an important vehicle for STEC transmission, and experimental evidence shows that STEC can colonize plants as secondary hosts, but differences in the capacity to colonize occur between different plant species and tissues. Therefore, an understanding of the impact that these plant factors have on the ability of STEC to grow and establish is required for food safety considerations and risk assessment. Here, we determined whether growth and the ability of STEC to form biofilms in plant extracts could be related to specific plant metabolites or could predict the ability of the bacteria to colonize living plants. Growth rates for sprouted seeds (alfalfa and fenugreek) but not those for leafy vegetables (lettuce and spinach) exhibited a positive relationship between plant extracts and living plants. Therefore, the detailed variations at the level of the bacterial isolate, plant species, and tissue type all need to be considered in risk assessment.
ruth
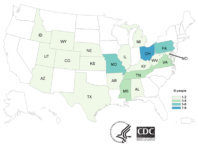
The Centers for Disease Control and Prevention (CDC) said that a multi-state salmonella outbreak is tied to contact with backyard poultry. This outbreak has made dozens of people sick out of 52 reported infections across 21 states, five people have been hospitalized. Salmonella serotypes involved in this outbreak are Salmonella Braenderup and Salmonella Montevideo. Illnesses started on dates from January 12, 2019, to April 29, 2019. WGS of four isolates from ill people predicted antibiotics resistance to amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftriaxone, or tetracycline. An additional five isolates from ill people did not show evidence of antibiotic resistance. Of 33 ill people interviewed, 23 (70%) reported contact with backyard poultry before becoming ill. Ill people reported buying poultry from various sources, including agricultural stores, websites, and hatcheries. Backyard poultry from multiple hatcheries are the likely source of these outbreaks. Regardless of where poultry was purchased, these birds can carry Salmonella that can make people sick. @ https://www.cdc.gov/salmonella/backyardpoultry-05-19/index.html
Outbreaks of Salmonella Infections Linked to Backyard Poultry