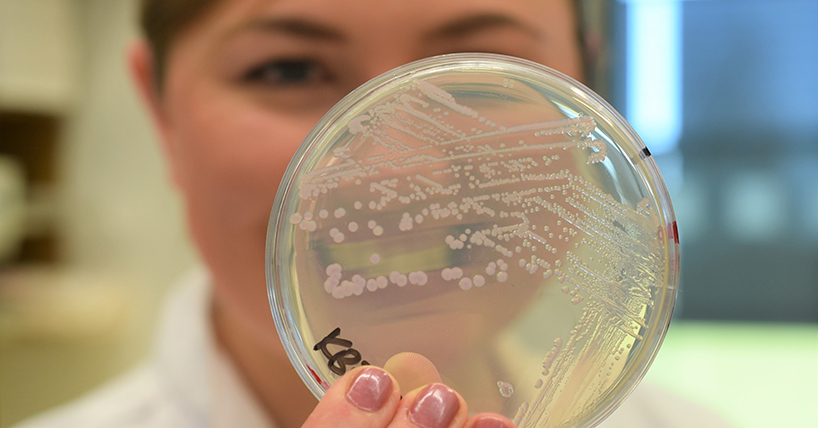
The U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced that Tip Top Poultry, Inc (Rockmart, GA) recalled an undetermined amount of ready-to-eat (RTE) poultry products that may be contaminated with Listeria monocytogenes. The frozen cooked, diced or shredded, RTE chicken products were produced between January 21, 2019, and September 24, 2019. These items were shipped to institutions nationwide in the United States and Canada. The problem was discovered when Tip Top notified FSIS that multiple samples were positive for the presence of Listeria monocytogenes after being tested in Canada. The firm decided to recall all cooked, diced or shredded, RTE chicken products produced from January 21, 2019 through September 24, 2019. Tip Top expanded the dates and the scope of the recall out of an abundance of caution. There have been no confirmed reports of adverse reactions due to consumption of these products. @ https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2019/recall-094-2019-release
ruth
Tip Top Poultry, Inc, a Rockmart, Ga., establishment, is recalling an undetermined amount of ready-to-eat (RTE) poultry products that may be adulterated with Listeria monocytogenes.
ruth
Maryland Department of Health is investigating a cluster of illnesses that involves “individuals who all reported eating at Moby Dick House of Kabob restaurant.” Since Sept. 10, eight of the nine confirmed cases of salmonella infections reported eating hummus from Moby Dick House of Kabob, which has several locations in Maryland, Virginia, and D.C. The state Health Departments of Virginia and DC did not report an investigation. The restaurant chain has voluntarily stopped the sale of its hummus. Sample of Hummus send by the restaurant to a third-party laboratory tested negatively for Salmonella. @ https://wtop.com/local/2019/09/salmonella-infections-linked-to-hummus-from-local-kabob-chain/
The Maryland Department of health is investigating a cluster of salmonella infections that have been linked to a local restaurant chain.
ruth
Scientists at Newcastle University have shown for the first time that bacteria can change form to avoid being detected by antibiotics in the human body. The team showed that bacteria could lose its cell wall that is the common target of many groups of antibiotics. They showed that bacteria could survive without a cell wall (known as “L-form switching”). The research shows that when antibiotics are present—such as in a patient with a UTI receiving penicillin or other cell wall-targeting antibiotic—then the bacteria can change form, losing the cell wall which is often the target of the antibiotic. In a previous publication, the team demonstrated that our immune system could also, to some extent induce L-form switching. The current study showed that L-forms of various bacterial species typically associated with UTIs including E. coli, Enterococcus, Enterobacter and Staphylococcus were detectable in 29 out of 30 patients involved in the study. The research also captured on video for the first time, L-form bacteria isolated from a patient with UTI re-forming a cell wall after the antibiotic had gone. @ https://www.ncl.ac.uk/press/articles/latest/2019/09/causeofantibioticresistanceidentified/
Scientists have confirmed for the first time that bacteria can change form to avoid being detected by antibiotics in the human body.
ruth
The FDA is advising pet owners not to feed their pets any Performance Dog frozen raw pet food due to a sample that tested positive for Salmonella and Listeria monocytogenes. Two samples of different finished products collected during an inspection of Bravo Packing, Inc. in Carneys Point, NJ, the manufacturer of Performance Dog raw pet food, tested positive for Salmonella and/or L. monocytogenes. Performance Dog raw pet food, lot code 072219, sold to customers frozen in two-pound pouches entered the market. The FDA is cautioning about all Performance Dog frozen raw pet food produced on or after July 22, 2019. Bravo Packing, Inc. had recalled products due to Salmonella in 2018.Bravo Packing, Inc. Performance Dog products are sold frozen in two-pound plastic pouches. @ https://www.fda.gov/animal-veterinary/news-events/fda-cautions-pet-owners-not-feed-performance-dog-raw-pet-food-due-salmonella-listeria-monocytogenes?utm_campaign=9-26-2019-PerformanceDog&utm_medium=email&utm_source=Eloqua
FDA is cautioning pet owners not to feed Performance Dog frozen raw pet food after a sample tested positive for Salmonella and/or L. mono.