U.S. PIRG (Public Interest Research Group) Education Fund is an independent, non-partisan group that works for consumers and the public interest published a new report about food recall at grocery stores. The report indicates that most grocery stores that could be a major place to learn about recalls don’t help consumers to uncover recall information. 84% of the nation’s 26 largest supermarket chains got a failing grade, including Walmart, Aldi, and Publix. Only Harris Teeter, Kroger, Smith’s and Target received a passing grade. Not a single store provided information online about recall and where they are posted (at customer service desks, checkout counters, or store shelves). U.S. PIRG Education Fund Consumer Watchdog Associate Dylan Robb said, “Stores might not be responsible for the recall, but they can make a difference.” Grocery retailers are in a unique position at the final point in the supply chain before the customer takes possession of food, and therefore have the opportunity to have a powerful impact on public safety. @ https://uspirg.org/news/usp/new-investigation-supermarkets-failing-warn-public-about-food-recalls
Emmy-S
Americans are not hearing about food recalls, and that communication breakdown is having serious repercussions for public health. A new report finds that most grocery stores — which should be one of the best places to learn about recalls — don’t make it easy for consumers to uncover this information.
ruth
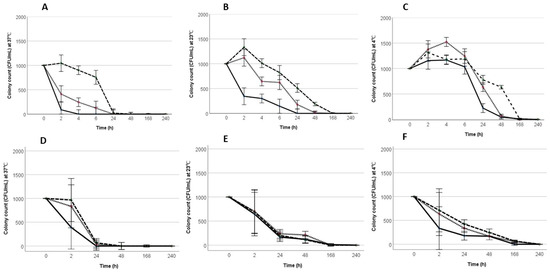
A study published in Pathogens (2019, 8(4), 218) describes the work of a research team from Flinders University in Adelaide, Australia, that developed a method to remove Salmonella Typhimurium (ST) from eggshells without effecting the egg’s usability and quality. The research investigated the effect of pH and temperature on the survival of Salmonella Typhimurium (ST) in peptone water (PW) and mayonnaise. The pH of PW and mayonnaise was adjusted to 4.2, 4.4, and 4.6 using acetic acid and vinegar, respectively. The PW and mayonnaise were inoculated with ST and incubated at 37 °C, 23 °C, and 4 °C. The survival of Salmonella was determined using the drop plate method. Survival was significantly (p < 0.05) improved at 4 °C. In both mayonnaise and PW, following 24 h, there was no ST growth at pH 4.2. Resuscitation of ST was rapidly observed at 4 °C, while complete inactivation was observed at 37 °C at pH 4.2, 4.4, and 4.6 in both PW and mayonnaise. Lower temperatures protected ST from the bactericidal effect of low pH. This study showed that lower temperatures reduce the antibacterial activity of the organic acids, which allowed Salmonella to survive at low pH for longer. Preparing home-made mayonnaise at pH 4.2 and retaining at room temperature for at least 24 h effectively killed S. Typhimurium and could aid in reducing the prevalence of the salmonellosis outbreaks in Australia. Results of this study indicate that the combined effect of pH 4.2 and the incubation temperature, 37 °C was the most effective at reducing S. Typhimurium; however, incubating at 23 °C was still more effective than 4 °C and may present a more practical approach. @ https://www.mdpi.com/2076-0817/8/4/218/htm
Raw egg products are often associated with salmonellosis. The Australian guidelines recommend raw egg mayonnaise to be prepared and stored under 5 °C and adjusted to a pH less than 4.6 or 4.2. Despite these guidelines, a significant amount of salmonellosis outbreaks are recorded annually in Australia. The aim of this study was to investigate the effect of pH and temperature on the survival of Salmonella Typhimurium (ST) in peptone water (PW) and mayonnaise. The pH of PW and mayonnaise was adjusted to 4.2, 4.4 and 4.6 using acetic acid and vinegar, respectively. The PW and mayonnaise were inoculated with ST and incubated at 37 °C, 23 °C, and 4 °C. The survival of Salmonella was determined using the drop plate method. Survival was significantly (p < 0.05) improved at 4 °C. In both mayonnaise and PW, following 24 h, there was no ST growth at pH 4.2. Resuscitation of ST was rapidly observed at 4 °C while complete inactivation was observed at 37 °C at pH 4.2, 4.4, and 4.6 in both PW and mayonnaise. Lower temperatures protected ST from the bactericidal effect of low pH. “The preparation of mayonnaise at pH 4.2 or less and incubating it at room temperature for at least 24 h could reduce the incidence of salmonellosis”.
ruth
Blendtopia Products, LLC is recalled 29,078 cases of 7 ounce frozen Blendtopia brand Superfood Smoothie Kits due to potential contamination with Listeria monocytogenes. The smoothie blends affected include Blendtopia brand “Glow,” “Detox,” “Energy,” “Immunity,” and “Strength” Superfood Smoothie Kits. The products were distributed nationwide and are sold at select retailers and online.
The problem was discovered during the quality control processes. The issue is believed to be isolated to a supplied ingredient. There have been no reports of sickness or illness to date associated with any consumption of products related to this recall. No other products made by Blendtopia Products, LLC, are affected by the recall. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/blendtopia-products-llc-voluntarily-recalls-frozen-smoothie-products-due-possible-health-risk
Blendtopia Products, LLC is voluntarily recalling 29,078 cases of 7 ounce frozen Blendtopia brand superfood Smoothie Kits because of potential contamination with Listeria monocytogenes.
ruth
Nuts ‘N More LLC from East Providence, RI recalled 4143 plastic jars of Plain Peanut Spread due to Listeria species. The product was distributed to locations in VA, AZ, MA, RI, ME, AL, IN, FL, as well as Canada and the UK. No complaints of illness have been reported to date. Routine testing performed by a 3rd Party Laboratory revealed the presence of Listeria species. The company has ceased the production and distribution of this product as the State of Rhode Island and the company continues its investigation. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/nuts-n-more-llc-recalls-plain-peanut-spread-because-possible-health-risk
Nuts ‘N More of East Providence, RI. is recalling 4143 jars of plain Peanut Spread because it has the potential to be contaminated with Listeria species and to protect the public from a potential health hazard.