Food Safety News reports that researchers at Purdue University developed a treatment that infuses metal surfaces with naturally occurring antimicrobial peptides, creating surfaces that kill bacteria trying to attach to it. The technology applies primarily to food processing and cutting surfaces. The technique can reduce the risk of cross-contamination. The technology creates an oxidized metal surface with nanometer-wide and micrometer-deep cracks where antimicrobial peptides can be infused in these microscopic cracks with a simple wet process. The material stored in the cracks release over time, and the oxidation process also colors the material, which provides a visual indicator of the materials remaining antimicrobial resistance. The process works on stainless steel and titanium and can be used on a wide range of commercial metal alloys. The creators are now looking for partners to commercialize their technology. @ https://www.foodsafetynews.com/2020/08/new-technology-creates-hard-metal-surfaces-that-kill-bacteria/
Dan-W
A treatment to infuse hardened metal surfaces with naturally occurring antimicrobial peptides has been developed by researchers at Purdue University. In
ruth
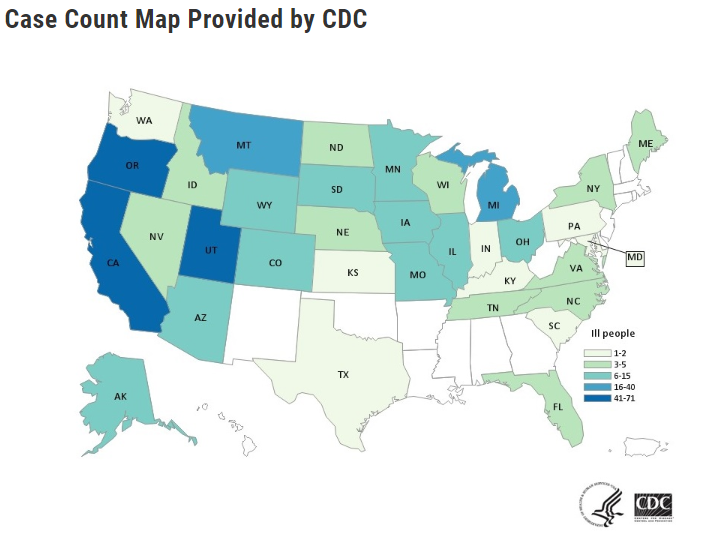
For several weeks an outbreak of Salmonella Newport is casing illnesses in numerous states in the US and Canada. The FDA and CDC had difficulty identifying the source. The outbreaks in the US and Canada have a genetic fingerprint closely related to each other. Earlier Friday, the Public Health Agency of Canada announced a recall by Sysco of its red onions because traceback work in that country showed them to be related to the outbreak there. Based on the Canadian information, and epidemiologic data on the US outbreak from CDC, the FDA’s traceback investigation identified Thomson International (Bakersfield, CA), Inc. red onions as the potential source of the infection. In the US, the outbreak caused 398 illnesses, 50 hospitalizations, and no death. States with Cases: AK (6), AZ (14), CA (49), CO (10), FL (3), ID (5), IL (10), IN (2), IA (15), KS (1), KY (1), ME (4), MD (1), MI (23), MN (10), MO (6), MT (33), NE (5), NV (5), NY (4), NC (3), ND (5), OH (7), OR (71), PA (2), SC (1), SD (11), TN (5), TX (1), UT (61), VA (4), WA (2), WI (5), WY (11). The Public Health Agency of Canada had found another 114 cases with those illnesses reported becoming ill after eating red onions at home, in menu items ordered at restaurants, and in residential care settings. No deaths have been reported in Canada. Thomson International will recall all varieties of its onions that could have contacted potentially contaminated red onions, due to the risk of cross-contamination. @ https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-salmonella-newport-red-onions-july-2020?utm_campaign=OutbreakAlert_SalmonellaOnion_07312020&utm_medium=email&utm_source=Eloqua
Do not eat, sell, or serve onions from Thomson International, Inc.
ruth
According to Canada Health Officials, as of July 30, 2020, there have been 114 confirmed cases of Salmonella Newport illness linked to this outbreak in the following provinces: British Columbia (43), Alberta (55), Manitoba (13), Ontario (2), and Prince Edward Island (1). The individual from Prince Edward Island reported traveling to Alberta before becoming ill. Saskatchewan has not reported any confirmed illnesses related to this outbreak, but provincial public health authorities are investigating some Salmonella Newport illnesses in the province. Individuals became sick between mid-June and mid-July 2020. Information is available for 102 illnesses. Out of 102 people, 16 individuals have been hospitalized. No deaths have been reported. Individuals who became ill reported eating red onions at home, in menu items ordered at restaurants, and in residential care settings. The US CDC is also investigating an outbreak of Salmonella Newport illnesses that have a similar genetic fingerprint to illnesses reported in this outbreak. The United States has been silent since July 24, 2020. As of July 23, 2020, a total of 212 people infected with the outbreak strain of Salmonella Newport have been reported from 23 states. At this time, the investigation has not identified a specific food, grocery store, or restaurant as the source of this outbreak. CDC will provide more information as it becomes available. @ https://www.foodpoisonjournal.com/foodborne-illness-outbreaks/canadian-health-officials-out-united-states-red-onions-as-cause-of-salmonella-newport-outbreak-united-states-health-officials-are-silent/
According to Canada, as of July 30, 2020, there have been 114 confirmed cases of Salmonella Newport illness linked to this outbreak in the following
Dan-W
The FDA announced a new protocol for the development and registration of antimicrobial treatments for pre-harvest agricultural water, such as the water used in farm irrigation systems. The FDA and EPA developed the protocol that might help farmers address contamination issues in their water sources and protect consumers from foodborne illness. The goal is to develop treatment products for the agricultural water used to irrigate our nation’s leafy greens. EPA’s approval of this protocol allows for companies to develop data on the effectiveness of their products in inactivating foodborne bacteria, such as E. coli or Salmonella, in pre-harvest agricultural water. While farmers are not required to treat their agricultural water, these treatments could be a valuable tool to help farmers ensure the safety of their produce. There currently are no registered antimicrobial treatment products that are authorized for use on agricultural fields, or for treatment of irrigation water systems or ponds. The FDA intends to release a proposed rule in late 2020, to revise certain agricultural water requirements in the Produce Safety Rule and to address practical implementation challenges while protecting public health. @ https://www.fda.gov/news-events/press-announcements/fda-announces-new-protocol-development-and-registration-treatments-preharvest-agricultural-water?utm_campaign=073020_PR_New%20Protocol%20Announced%20for%20Treatments%20for%20Preharvest%20Agricultural%20Water&utm_medium=email&utm_source=Eloqua
FDA Announces New Protocol for Treatments for Preharvest Agricultural Water