As a result of the recall by Freshouse Produce Wegmans Food Markets, Inc. (with stores in New York, Pennsylvania, New Jersey, Virginia, Maryland, Massachusetts, and North Carolina) is recalling its four-pound bag of Valencia Oranges, two-pound bag of lemons, bulk lemons, and a variety of in-store produced seafood and restaurant foods items that contain fresh lemon because they have the potential to be contaminated with Listeria monocytogenes. The affected products were sold in Wegmans stores in New Jersey, Pennsylvania, Virginia, North Carolina, Maryland, and Brooklyn, and Harrison, NY. The products were sold between July 31 and August 7, 2020, include Wegmans 4lb Bag Valencia Oranges – UPC: 7789052363; Wegmans 2lb Bag Lemons – UPC: 7789015917; and Wegman’s bulk lemons – UPC: 4033. No illnesses associated with this recall have been reported to Wegmans or its supplier. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/wegmans-food-markets-inc-announces-recall-select-valencia-oranges-lemons-and-various-products?utm_campaign=Wegmans%20Food%20Markets%2C%20Inc.%20Announces%20Recall%20of%20Select%20Valencia%20Oranges%2C%20Lemons%2C%20and%20Various%20Products&utm_medium=email&utm_source=Eloqua
ruth
Wegmans Food Markets, Inc. is recalling its four-pound bag of Valencia Oranges, two-pound bag of lemons, bulk lemons, and a variety of in-store produced seafood and restaurant foods items that contain fresh lemon because they have the potential to be contaminated with Listeria monocytogenes, an orga
ruth
Freshouse II, LLC of Salisbury, NC recalled specific production lots, of Valencia Oranges, Lemons, Limes, Organic Limes, and Red B Potatoes because they have the potential to be contaminated with Listeria monocytogenes. The recall initiated after the company’s routine internal testing identified Listeria monocytogenes on a piece of equipment in one of our packing facilities. The company has ceased the production and distribution of the products that was packed on the equipment in question and are taking corrective actions and continually evaluating our cleaning and sanitation regimes. No illnesses have been associated with this recall to date. Freshouse II has notified its retail and wholesale customers who received the recalled product directly from the company and is requesting that these customers remove the recalled product from commerce. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/freshouse-ii-llc-voluntarily-recalls-select-mesh-bags-and-bulk-shipments-potatoes-limes-valencia
Freshouse II, LLC of Salisbury, NC is recalling the following specific production lots, brands and weights of Valencia Oranges, Lemons, Limes, Organic Limes, and Red B Potatoes because they have the potential to be contaminated with Listeria monocytogenes, an organism which can cause serious and som
ruth
The USDA-FSIS issued an alert for Bluegrass Provisions Co., (Crescent Springs, KY) that produced sausage products that may be contaminated with Listeria monocytogenes. A recall was not requested because it is believed that all products are no longer in commerce and are past their use or freeze by dates. The products include BLUE GRASS METTWURST, WALNUT CREEK FOODS Smoked Sausage, SMOKED BRATWURST, and SMOKED BRATWURST WITH CHEESE. The products were shipped to distributors and retail locations in Kentucky, Ohio, North Carolina, and Virginia. The problem was discovered by routine testing, and the results showed one of the products was contaminated with Listeria monocytogenes. The additional products may be affected by cross-contamination. FSIS is concerned that some product may be in consumers’ freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase. FSIS advises all consumers to reheat ready-to-eat products until steaming hot. @ https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/current-recalls-and-alerts/!ut/p/a1/jY_BCoJAGISfxQdY9jdF9CgLlpa7iGS2l1hMTdhWWa1DT5_SyUhy_tPAN_MzmOMccyWeTS2GplVCTp47F0jAMT0CEQv8AEJqBZlLtyYwZwTOM8AzJyBL2J4QcKm1Mr8gH_7loxUPNjomcY15J4YbalTV4rx4aF2qAemyEFL2SKgrErLUQ49PmM87wRxv7EztXUQtYPY38GP0B1he1d2P-etQpWFtGG85uhP2/?1dmy¤t=true&urile=wcm%3apath%3a%2Ffsis-content%2Finternet%2Fmain%2Fnewsroom%2Fnews-releases-statements-and-transcripts%2Fnews-release-archives-by-year%2Farchive%2F2020%2Fpha-08072020-01
ruth
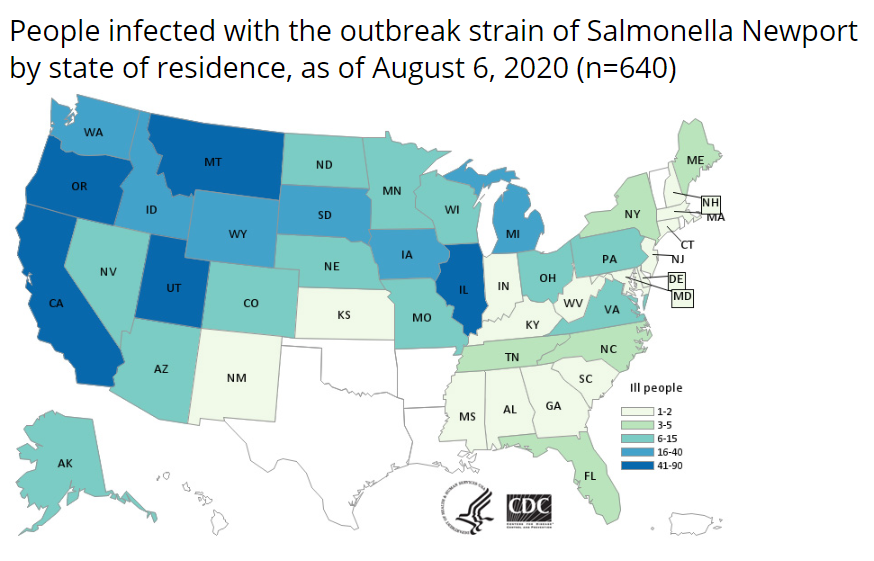
The CDC reported that as of August 6, 2020, a total of 640 people infected with the outbreak strain of Salmonella Newport had been reported from 43 states. This represents an increase of 250 cases in several days. The US cases are located in Alaska (6), Alabama (1), Arizona (14), California (76), Colorado (14), Connecticut (2), Delaware (1), Florida (3), Georgia (1), Idaho (26), Illinois (41), Indiana (2), Iowa (20), Kansas (2), Kentucky (1), Maine (4), Maryland (1), Massachusetts (2), Michigan (36), Minnesota (14), Mississippi (2), Missouri (6), Montana (52), Nebraska (10), Nevada (8), New Hampshire (1), New Jersey (2), New Mexico (1), New York (5), North Carolina (5), North Dakota (8), Ohio (8), Oregon (85), Pennsylvania (9), South Carolina (1), South Dakota (17), Tennessee (5), Utah (90), Virginia (8), Washington (25), West Virginia (2), Wisconsin (7), and Wyoming (16). Of 343 ill people with information available, 85 hospitalizations have been reported. No deaths have been reported. The onions have various labels, including Thomson Premium, TLC Thomson International, Tender Loving Care, El Competitor, Hartley’s Best, Onions 52, Majestic, Imperial Fresh, Kroger, Utah Onions, and Food Lion. Some foods made with recalled onions, such as deli salads and vegetable mixes, have also been recalled. Publix Super Markets (red onions, packaged by Del Monte Fresh Produce, NA, Inc. and sold in bulk merchandise displays in AL, GA, NC, SC, TN, and VA). Giant Eagle (red, yellow, and white onions sold in produce departments in PA, OH, WV, IN, and MD). Taylor Farms recalled products. Public Health Agency of Canada (PHAC) reported that as of August 7, 2020, there have been 239 confirmed cases of Salmonella Newport illness linked to this outbreak in the following provinces: Alberta (149), British Columbia (67), Manitoba (13), Saskatchewan (5), Ontario (3), Quebec (1), and Prince Edward Island (1). @ https://www.cdc.gov/salmonella/newport-07-20/index.html#:~:text=CDC%2C%20public%20health%20and%20regulatory,Newport%20infections%20linked%20to%20onions.&text=Do%20not%20eat%2C%20serve%2C%20or,food%20made%20with%20these%20onions.
Food Safety Alert: A multistate outbreak of Salmonella Newport infections has been linked to onions.