Health Canada announced on its website that National Herring Co. is recalling Adar brand Schmaltz Herring in Oil from the marketplace due to possible Listeria monocytogenes contamination. This recall was triggered by the Canadian Food Inspection Agency (CFIA) test results. There have been no reported illnesses associated with the consumption of this product. @ https://healthycanadians.gc.ca/recall-alert-rappel-avis/inspection/2020/74127r-eng.php
ruth
National Herring Co. is recalling Adar brand Schmaltz Herring in Oil from the marketplace due to possible Listeria monocytogenes contamination. Consumers should not consume the recalled product described below.
ruth
The FDA announced on its website that Red Monkey Foods, Inc. voluntarily recalled select organic parsley as part of a recall initiated by High Quality Organics (HQO). HQO has issued a recall for a lot of parsley because a sample was tested by one of HQO’s customers and was found to be potentially contaminated with Salmonella. A portion of the lot recalled by HQO was supplied to Red Monkey Foods, Inc and subsequently repacked into consumer containers for parsley and was also used to manufacture herbes de provence, which was then sold in consumer containers. To date, there have been no consumer complaints or reported cases of Salmonellosis in connection with these products. The affected product consists of retail packages weighing 0.3-0.65 oz. of Cost Plus World Market Herbes De Provence, Cost Plus World Market Organic Parsley, Great Value Herbes De Provence Organic, Great Value Organic Parsley Flakes, O Organics Herbes De Provence Organic, O Organics Parsley Organic, and Full Circle Parsley Organic. The products were distributed to all fifty states and Puerto Rico. The product was produced for sale at retail. There have not been any reported cases of Salmonellosis associated with the products. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/red-monkey-foods-inc-recalls-parsley-and-herbes-de-provence-because-possible-health-risk
Red Monkey Foods, Inc. out of an abundance of caution is voluntarily recalling select organic parsley as part of a recall initiated by High Quality Organics (HQO). HQO has issued a recall for a lot of parsley because a sample was tested by one of HQO’s customers and was found to be potentially conta
ruth
The Canadian Food Inspection Agency (CFIA) is warning the public not to consume and retailers, restaurants and institutions not to sell or use Alwatania (with Arabic characters only on the box) due to possible Salmonella contamination. The product has been distributed in Quebec and may have been distributed in other provinces or territories. There have been no reported illnesses associated with the consumption of this product. @ https://healthycanadians.gc.ca/recall-alert-rappel-avis/inspection/2020/74115a-eng.php
The Canadian Food Inspection Agency (CFIA) is warning the public not to consume and retailers, restaurants and institutions not to sell or use the product described below due to possible Salmonella contamination.
ruth
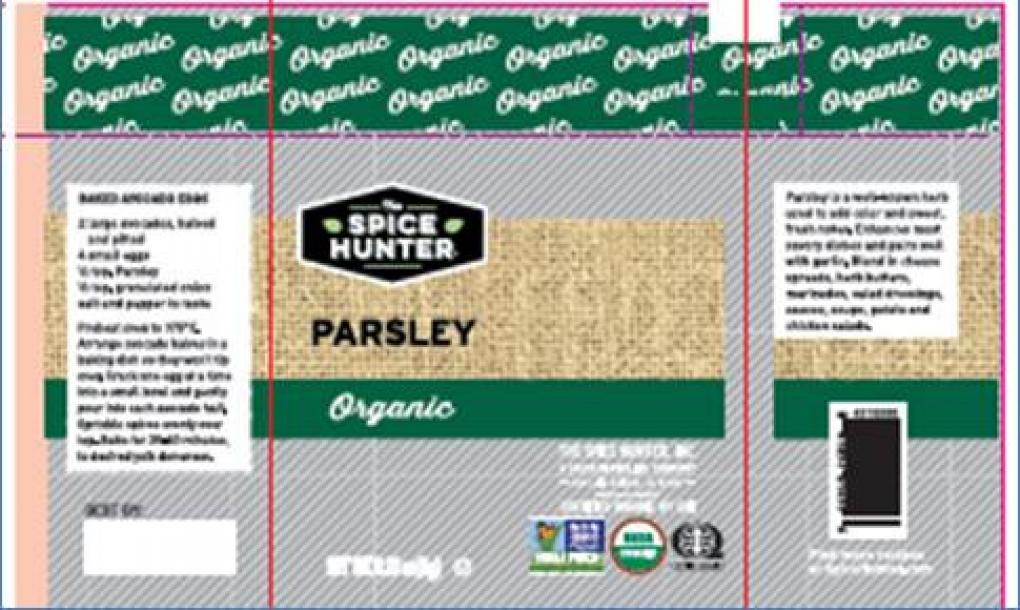
The FDA announced on its website that Sauer Brands, Inc. recalled several of The Spice Hunter Products due to the potential presence of Salmonella. After initially certifying that their raw material had tested negative for Salmonella, their supplier notified them of the potential presence of salmonella in specific lots of organic parsley that it provided to Sauer Brands. Those lots of parsley were used on two specific days of production. All other products produced on the same days are also being recalled out of an abundance of caution due to potential cross-contamination. The recalled products include Organic Parsley, Saigon Organic Cinnamon, Madagascar Cloves, Gourmet Sesame Seeds, Herbes De Provence, Pumpkin Pie Spice, Seafood Grill & Broil, Coriander, California Garlic, Green Hatch Chile, Mexican Seasoning, Black Pepper, Paprika, Szechwan Seasoning, Fine Black Pepper, Chinese Ginger, Muntock White Pepper, Roasted Garlic, Everything Bagel Crunch, Malabar Black Peppercorns, Freeze-Dried Chives, Italian Seasoning, Cilantro, Fennel Seeds, Dill Weed, Arrowroot, and Cayenne Red Pepper. The Spice Hunter Products in question were distributed to the states of Alaska, Alabama, Arizona, California, Colorado, Delaware, Florida, Georgia, Illinois, Indiana, Kansas, Kentucky, Louisiana, Maryland, Michigan, Missouri, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Utah, Virginia, Washington, and Wisconsin. The product was produced for sale at retail and spicehunter.com. These products come in clear glass jars. There are no reports of illnesses associated with this recall. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/sauer-brands-inc-voluntarily-recalls-certain-spice-hunter-products-because-potential-salmonella
Sauer Brands, Inc. is voluntarily recalling The Spice Hunter Products listed below due to the potential presence of Salmonella. After initially certifying that our raw material had tested negative for salmonella, and was fit for human consumption, our supplier notified us of the potential presence o