The FDA announced on its website that Vegpro International ( Sherrington, Quebec, Canada) recalled Fresh Attitude baby spinach (5oz and 11oz) with Best before dates of Dec 4th (for 11oz) and Dec 4th & 5th (for the 5oz), because it has the potential to be contaminated with Salmonella. These products were produced in Vegpro’s Eastern Canadian plant and distributed in Eastern Canada and the Northeastern United States (NY, NJ, DE, CT, MD & PA ). The product was possibly contaminated with Salmonella; the problem may have been caused by contamination of a part of a lot of Baby Spinach. No illnesses have been reported. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/vegpro-international-issues-recall-fresh-attitude-baby-spinach-because-potential-salmonella-health?utm_medium=email&utm_source=govdelivery
ruth
Vegpro International of Sherrington, QC CAN is recalling Fresh Attitude baby spinach (5oz and 11oz) with Best before dates of Dec 4th (for 11oz) and Dec 4th & 5th (for the 5oz), because it has the potential to be contaminated with Salmonella, an organism which can cause serious and sometimes fatal
ruth
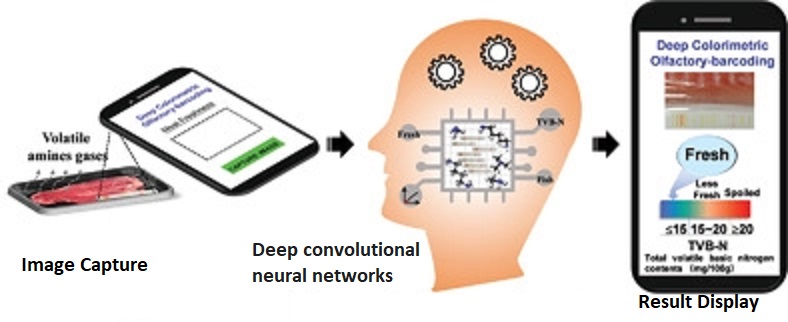
An article in Advance Materials (Vol 32, Issue 45, Nov 2020) reported developing an electronic nose (e-nose) that employs a colored barcode that reacts with gasses produced by decaying meat. The system also includes a cross-reactive colorimetric barcode reader, reactive to gasses produced by decaying meat that uses artificial intelligence (AI) to interpret the combination of colors on the barcode. A smart-phone application was integrated into the system yielding in 30 seconds. A fully supervised convolutional neural network (DCNNs) trained using 3475 labeled barcode images predicts meat freshness with an overall accuracy of 98.5%. The system is fast, accurate, and non‐destructive, enabling consumers and all stakeholders in the food supply chain to monitor food freshness. @ https://onlinelibrary.wiley.com/doi/10.1002/adma.202004805
ruth
On Saturday (Nov. 28), the Canadian Food Inspection Agency (CFIA) issued a notice that Metro Ontario Inc. recalled 21 Metro brand products due to possible contamination with Salmonella. The CFIA announced that the products include units sold up to and including Nov. 27 across Ontario. The products include salmon torenados, Cod Vegetable Roast, Tilapia Roast Stuffed Vegetable /Cheese, tropical green juice, Hawaiian Green Juice, spinach-fruit salad with nuts, and rainbow trout stuffed with vegetable and cheese. Metro Ontario Inc. triggered the recall and noted that no illnesses connected with the affected products’ consumption had been reported. @ https://www.inspection.gc.ca/food-recall-warnings-and-allergy-alerts/2020-11-28/eng/1606600071713/1606600071916
Metro Ontario Inc. is recalling certain Metro brand products from the marketplace due to possible Salmonella contamination.
ruth
The USDA-FSIS announced on its website that Tarantino Wholesale Foods Distributor (San Diego, California) recalled ~ 1,115 pounds of ready-to-eat chicken breast products due to undercooking, resulting in the potential survival of bacterial pathogens in the products. The ready-to-eat grilled chicken breast items were produced on October 23, 2020. These items were shipped to institution locations in California and were sold directly to retail consumers. Customer complaints that the ready-to-eat chicken product appeared to be undercooked triggered the recall. No illnesses were reported due to the consumption of this product. @ https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2020/recall-027-2020-release
Tarantino Wholesale Foods Distributor, a San Diego, Calif., establishment, is recalling approximately 1,115 pounds of ready-to-eat chicken breast products due to undercooking, resulting in the potential survival of bacterial pathogens in the products.