The FDA announced that MG Foods of Charlotte, NC, recalls various Turkey Sandwiches due to potential contamination of Listeria monocytogenes. The products were distributed between March 3, 2021, and March 5, 2021, and packaged in transparent plastic wedges & paper bags. The products were sold at distributors located at the Charlotte Douglas Airport and via vending machines and micro-markets located in business locations in Georgia, North Carolina, South Carolina, and West Virginia. No illnesses have been reported to date.MG foods detected the presence of Listeria on surface areas where the recalled products were produced on March 2, 2021, during routine environmental testing. At the Charlotte Douglas Airport, MG Foods Combo Half & Half, MG Foods Turkey & Cheddar BLT, and MG Foods Turkey & Swiss Croissant were recalled. Products recalled in vending machines and micro markets located in Georgia, North Carolina, South Carolina and West Virginia include Fresh to You Club on Toast, Fresh to You Club Panini, Fresh to You Club Sub, Fresh to You Deluxe Triple Decker Club, Fresh to You Ham & Turkey Combo, Fresh to You Ham & Turkey Combo on 12 Grain, Fresh to You Jumbo Turkey & Cheese Sub, Fresh to You Market Club, Fresh to You Market Ham & Turkey Combo, Fresh to You Market Shaved Turkey & Cheese, Fresh to You Shaved Turkey & Cheese, Fresh to You Turkey & Baby Swiss on a Honey Brown Roll, Fresh to You Turkey & Cheddar Club, Fresh to You Turkey & Cheese Cut, Fresh to You Turkey & Cheese Hoagie, Fresh to You Turkey & Sun-Dried Tomato Aioli on Pita, Fresh to You Turkey & Swiss on a Kaiser Roll, Fresh to You Turkey & Swiss on a Wheat Roll, Fresh to You Turkey & Swiss on Whole Wheat, Fresh to You Turkey Club Croissant, Fresh to You Turkey Cranberry Pita, Fresh to You Turkey Melt Croissant, Fresh to You Tuscan Turkey Ciabatta, Combo Half & Half, The Club Sub, Turkey & Cheddar BLT, Turkey & Pepperjack Sub, Turkey & Provolone BLT, Turkey & Provolone on 12 Grain, and Turkey & Swiss Croissant. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/mg-foods-recalls-various-turkey-sandwiches-due-possible-listeria-monocytogenes-contamination?utm_medium=email&utm_source=govdelivery
MG Foods of Charlotte, NC is recalling various Turkey Sandwiches due to a potential contamination of Listeria monocytogenes.
ruth
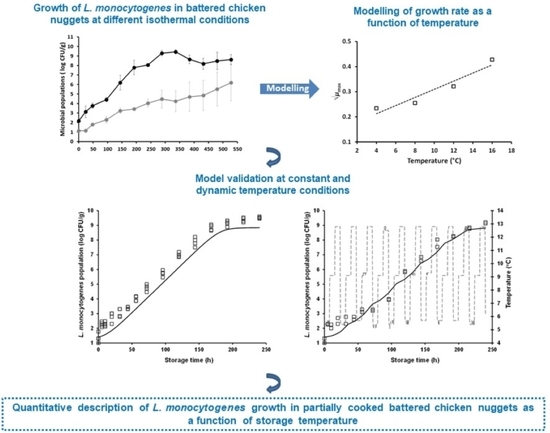
A study conducted by scientists from the Agricultural University of Athens, the University of Patras in Greece and North Carolina State University and published in Foods 2021, 10(3), 533 studied the growth of Listeria monocytogenes in chicken nuggets. Battered poultry products, such as chicken nuggets may be wrongly regarded as ready-to-eat. Commercially prepared chicken breast nuggets were inoculated with L. monocytogenes and stored at different isothermal conditions (4, 8, 12, and 16 °C). The study found that uncooked, frozen, commercially prepared chicken nuggets could potentially promote Listeria growth over the course of their shelf life. L. monocytogenes may grow in battered chicken nuggets to levels potentially hazardous even under well-controlled refrigerated storage conditions. @ https://www.mdpi.com/2304-8158/10/3/533
Battered poultry products may be wrongly regarded and treated by consumers as ready-to-eat and, as such, be implicated in foodborne disease outbreaks. This study aimed at the quantitative description of the growth behavior of Listeria monocytogenes in fresh, partially cooked (non-ready-to-eat) battered chicken nuggets as function of temperature. Commercially prepared chicken breast nuggets were inoculated with L. monocytogenes and stored at different isothermal conditions (4, 8, 12, and 16 °C). The pathogen’s growth behavior was characterized via a two-step predictive modelling approach: estimation of growth kinetic parameters using a primary model, and description of the effect of temperature on the estimated maximum specific growth rate (μmax) using a secondary model. Model evaluation was undertaken using independent growth data under both constant and dynamic temperature conditions. According to the findings of this study, L. monocytogenes may proliferate in battered chicken nuggets in the course of their shelf life to levels potentially hazardous for susceptible population groups, even under well-controlled refrigerated storage conditions. Model evaluation demonstrated a satisfactory performance, where the estimated bias factor (Bf) was 0.92 and 1.08 under constant and dynamic temperature conditions, respectively, while the accuracy factor (Af) value was 1.08, in both cases. The collected data should be useful in model development and quantitative microbiological risk assessment in battered poultry products.
ruth
The FDA announced on 3/9/2021 that it received confirmation that the cheeses were also distributed in Rhode Island. The states with confirmed distribution now include AL, CT, FL, GA, IA, IL, IN, KS, KY, MA, MD, MI, MN, MO, MS, NC, NJ, NY, NE, OH, PA, RI, SC, TN, VA, and WI. Recalled brands by cheese type include: Queso Fresco: El Abuelito, Rio Grande, Rio Lindo; Quesillo: El Abuelito, El Viejito, El Paisano, El Sabrosito, La Cima, Quesos Finos, San Carlos, Ideal Brands; Requeson: El Abuelito, El Viejito. Some of the cheeses were sold in bulk quantities and could have been repackaged by retailers. There have been a total of 11 Illnesses, 10 hospitalizations, and 1 death. States with Cases: CT (1), MD (4), NY (4), and VA (2). @ https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-hispanic-style-fresh-and-soft-cheeses-february-2021?utm_medium=email&utm_source=govdelivery
Do not eat, sell, or serve recalled Queso Fresco, Quesillo (Oaxaca, string cheese) or Requeson (ricotta) cheeses.