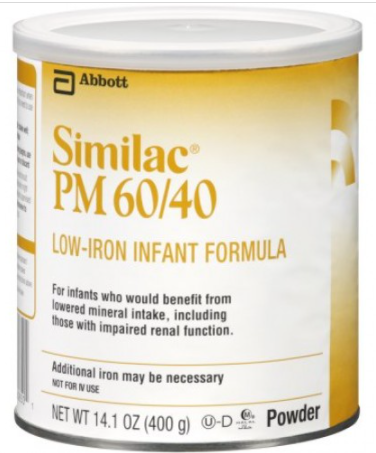
As of February 28, CDC has announced one additional illness of Cronobacter sakazakii with exposure to powdered infant formula produced at Abbott Nutrition’s Sturgis, MI facility. Cronobacter infection may have been a contributing cause of death for this patient. In total, this investigation includes four reports of Cronobacter sakazakii infections in infants (three from FDA complaints and one from a CDC case finding) and one complaint of a Salmonella Newport infection in an infant. All five illnesses resulted in hospitalization, and Cronobacter may have contributed to death in two patients. The most recent patient was reported to have consumed Abbott Nutrition’s Similac PM 60/40 product with the lot code 27032K800 before Cronobacter sakazakii infection. FDA and CDC informed Abbott of these findings, and on February 28, 2022, Abbott Nutrition voluntarily recalled Similac PM 60/40 powdered infant formula with the lot code 27032K800. This is a specialty formula for certain infants who would benefit from lowered mineral intake and was not included in the previous recall. This lot of Similac PM 60/40 was distributed to the U.S. and Israel. In total, four infants in Minnesota (1), Ohio (2), and Texas (1) consumed formula produced at the Sturgis, Michigan, before they got sick. These infants consumed formula that included Similac Sensitive, Similac Pro-total Comfort, Similac Advance, and Similac PM 60/40. @ https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigation-cronobacter-and-salmonella-complaints-powdered-infant-formula-february-2022?utm_medium=email&utm_source=govdelivery