The FDA posted on its website that “Real Water” did not cooperate with the authorities in their investigation. As a result, the investigators have been unable to complete the investigation at the “Real Water” facilities in Henderson, NV, and Mesa, AZ, because the company provided no records. As a result, on March 23, FDA issued a Demand for Records under section 414 of the Federal Food, Drug, and Cosmetic Act. Although the investigation is ongoing, current epidemiologic information indicates that this alkaline water product may cause illnesses. Currently, there are five illnesses associated with the product. @ https://www.fda.gov/food/outbreaks-foodborne-illness/investigation-acute-non-viral-hepatitis-illnesses-real-water-brand-alkaline-water-march-2021?utm_medium=email&utm_source=govdelivery
ruth
Do not drink, cook with, sell, or serve “Real Water” alkaline water
ruth
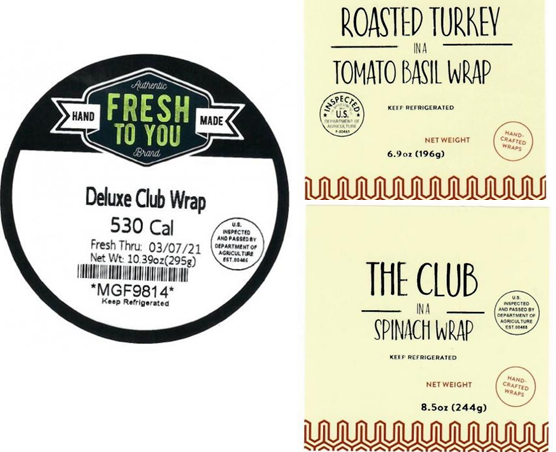
The FDA announced that MG Foods (Charlotte, NC) expanded the recall issued March 10, 2021, to include three Turkey Wraps due to potential contamination of Listeria monocytogenes. The three products are Fresh to You Deluxe Club Wrap, MG Foods Roasted Turkey in a Tomato Basil Wrap, and MG Foods The Club in a Spinach Wrap. The products were distributed between March 3, 2021, and March 5, 2021, and packaged in clear plastic wedges & plastic wrap. The products were sold exclusively via vending machines and micro-markets located in business locations in Georgia, North Carolina, South Carolina, and West Virginia. The company reacted quickly, and as of the end-of-day March 5, 2021, affected products were removed from sale at all locations. No illnesses have been reported to date. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/three-turkey-wrap-sandwiches-added-mg-foods-previous-recall-list-due-possible-listeria-monocytogenes
MG Foods of Charlotte, NC is expanding its recall issued March 10, 2021 to include three Turkey Wraps due to potential contamination of Listeria monocytogenes. Listeria monocytogenes is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and
ruth
The FDA, CDC, and state and local partners investigate reports on acute non-viral hepatitis in Nevada traced to “Real Water.” Real Water Inc. is headquartered in Mesa, Arizona. The company did not initiate a recall. However, health officials are warning the public not to drink the water. The health district in Las Vegas received initial reports of the five cases of acute non-viral hepatitis in November 2020. The patients include three adults and three children. The water was distributed in four states in 5-gallon bottles via home delivery subscriptions and nationwide in smaller containers via online sales. The 5-gallon containers are delivered to homes in the following areas: Honolulu, HI through Aloha Water, Orange County, CA through Paradise Bottling Company, St. George, UT through Real Water Southern Utah, Tucson, AZ through Aqua-Pure, and Ventura and Santa Barbara, CA through Real Water Gold Coast. Additionally, according to the firm’s website, Real Water is sold in 1 gallon, 500 mL (16.9oz.), 1 liter, and 1.5-liter plastic bottles and a 750 ml glass bottle. Acute non-viral hepatitis is an inflammation of the liver that can be caused by exposure to toxins. @ https://www.fda.gov/food/outbreaks-foodborne-illness/investigation-acute-non-viral-hepatitis-illnesses-real-water-brand-alkaline-water-march-2021?utm_medium=email&utm_source=govdelivery
Do not drink, cook with, sell, or serve “Real Water” alkaline water
ruth
The FDA announced that PNHC, LLC, d/b/a Heal the World (Raleigh, North Carolina), is voluntarily recalling all lots of Heal the World hand sanitizer packaged in 9.6 fl. oz containers to the consumer level. The products are being recalled because they resemble 9.6ounce water bottles. Drinking hand sanitizer could potentially result in alcohol toxicity. To date, PNHC, LLC, d/b/a Heal the World has received no reports of adverse reactions, and no complaints have been received. The product was distributed to selected retailers in the United States. PNHC, LLC has provided notification to its distributors and retailers. @ https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pnhc-llc-dba-heal-world-issues-voluntary-nationwide-recall-heal-world-hand-sanitizer-packaged-96?utm_medium=email&utm_source=govdelivery
PNHC, LLC, d/b/a Heal the World, is voluntarily recalling all lots of Heal the World hand sanitizer packaged in 9.6 fl. oz containers to the consumer level. The products are being recalled because they resemble 9.6ounce water bottles. The recall does not affect any other Hand Sanitizer products f