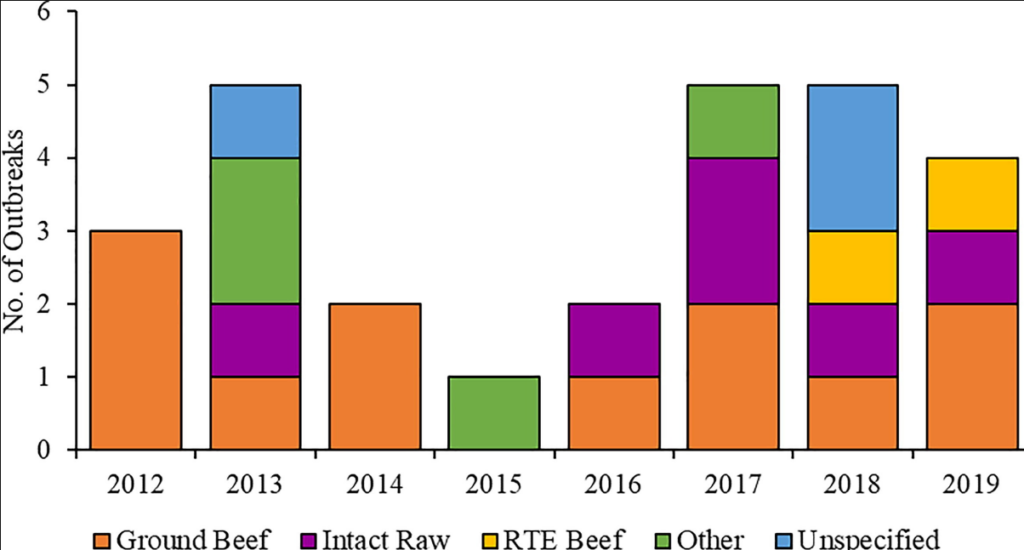
CDC published an article (in J. Food Protec 2023, (4)) on the prevalence of Salmonella in beef products. The CDC has identified nontyphoidal Salmonella as one of the top five pathogens contributing to foodborne illnesses in the United States. Despite implementing interventions at slaughter and processing facilities to reduce contamination of beef, Salmonella remains the main reason for outbreaks in the United States during 2012–2019. During 2012–2019, 27 Salmonella outbreaks were linked to beef consumption, resulting in 1,103 illnesses, 254 hospitalizations, and two deaths. The most common category of beef implicated was non-intact raw (12 outbreaks, 44%), followed by intact raw (6 outbreaks, 22%). Ground beef was responsible for the most illnesses (800, 73%). Both reported deaths and were the source of the largest outbreak. Antimicrobial Resistance data were available for 717 isolates from 25 (93%) outbreaks. Nine (36%) of these outbreaks had isolates resistant to one or more antibiotics, of which eight (89%) contained multidrug-resistant isolates. Several reported outbreaks highlight challenges during investigations, areas where further research may be warranted, and opportunities to prevent future outbreaks along the farm-to-fork continuum. @ https://www.sciencedirect.com/science/article/pii/S0362028X23067431#s0020