FSMA and Pathogen Environmental Monitoring (PEM) Programs: Where are we?
 Environmental monitoring is required under CFR 21 section 117.165 and under the FDA Final Rule for Preventive Controls for Human Food. Its purpose is to verify the implementation and effectiveness of the preventive controls put in place. Most food companies need to comply with these rules now or in the very near future.
Environmental monitoring is required under CFR 21 section 117.165 and under the FDA Final Rule for Preventive Controls for Human Food. Its purpose is to verify the implementation and effectiveness of the preventive controls put in place. Most food companies need to comply with these rules now or in the very near future.
Purpose of the PEM Program
The reason for having a PEM program is to identify problematic areas in the manufacturing facility where pathogens can harbor and become a source of contamination (“niches”), and assess the effectiveness of the sanitation programs.
Environmental monitoring may include pathogens or indicator organisms, depending on the products being manufactured. The program should encourage finding locations that will yield positive pathogen results, with the idea that “If it’s there and you don’t find it, FDA will.”
Another goal of the program is to prevent cross contamination or recontamination of the product from the environment. Positive results need to be followed with root-cause analysis, and the results need to be used immediately for corrective actions as well as long term improvements.
The Zone Concept
Zones are defined based on the probability of product contamination.
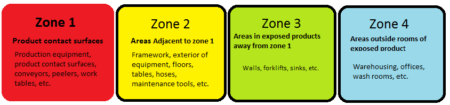
If a pathogen is present in environmental samples from zone 1, it is likely to contaminate the product. Food contact surfaces are usually not sampled forSalmonellae. In contrast, it is essential to test food contact areas for Listeria monocytogenes.
Zone 2 is the main focus for PEM investigations of Salmonellae since it is where environmental contamination is most likely to influence the safety of the product.
Zone 3, if contaminated with a pathogen, could lead to contamination of Zone 2 via actions of humans or movement of machinery.
Zone 4, if contaminated with a pathogen, could lead to contamination of Zone 3 via the actions of humans or machinery.
Any pathogen detection in zones 1-3 needs to be addressed by a documented corrective action.
When and Where to Test
When: Pre-op areas are less likely to yield positive samples. However, if found it is more easy to interpret and will identify sanitation weaknesses.
Three to four hours after the initiation of processing, it is more likely to find swabs that are positive for pathogens. It might provide information on the spread of the pathogen during processing. Site in which pathogens are detected requires pre-op follow-up sampling to identify pathogen source/niche.
Where to test: In niches such as hollow rollers, table legs, floor wall junctures, floor cracks, areas that are difficult to clean, seals on doors, transfer points such as door handles, and operator’s hands.
What are Reported FDA Actions?
For facilities that produce Ready-to-Eat products, it is common for the FDA to perform intensive swabbing of the manufacturing site, termed by some “swab-a-thon.” The team of FDA investigators will take samples in hard to reach areas, which are difficult to sanitize.
The FDA investigators are instructed to take hundreds of samples, focusing on Salmonella swabbing in Zones 2 and also collect a few samples in zones 3 and 4; The Listeria swabbing focus is on zones 1 and 2. In some recent swab-a-thons numerous samples were collected in Zones 3 and 4, and positive pathogen findings in these areas led to some recalls.
One goal of the program is to sequence the environmental pathogens found and compare them to stains that have caused illnesses. Another goal is to find “persistent” strains that are persisting in the manufacturing environment. Finding such strains could suggest that the corrective action is inadequate, and could result in a recall. Alternatively, it could mean that the same strains are re-introduced to the facility due to raw ingredients.
Many recalls have resulted from detection of pathogens in the environment. With whole genome sequencing Listeria outbreaks can be detected when only two people have gotten ill from a certain product. Finding that the same strain of Listeria is making people sick is an indication that these illnesses may have originated from the same source. Additionally, positive samples resulting from these swab-a-thons may cause costly product recalls, and a cease in operations until the pathogen has been eliminated. Also, the positive sample’s DNA will be cross-referenced with the PulseNet Database to see if it matches a strain linked to an illness.
Seek and Destroy
The complete elimination of L. monocytogenes from post processing contamination is difficult because: (i) this pathogen is common in various environments outside processing plants; (ii) it can endure in food processing environments for years.
Essentially, the “seek and destroy” process is to disassemble the equipment, and during disassembly, to evaluate the equipment for growth niches. If growth niches are discovered during the process, a microbiological assessment of those areas is conducted.
Because of the impact of a pathogen being found in Zone 1, it is preferred to comprehensively and systematically monitor Zones 2 and 3. These areas should be tested with the end goal to seek and destroy any pathogen present. This approach requires digging into hard-to-reach places and look for problems. Seek to find and destroy any issues in Zones 2 and 3 before they can hit Zone 1.
If a pathogen or indicator organism is found in Zone 2 or 3, the faster it is detected and corrected, the less likely it is to have implicated product. This call for a systematic approach to finding niches in the food processing plant where growth can occur, and either eradicate or mitigate effects of these niches.
Need for Rapid Testing Methods
The faster results can be obtained after sample collection, the quicker you can destroy the potential of food contact surface contamination, and prevent the potential of product contamination.
Using newer and faster molecular based technology for detection, rather than traditional 48-hour or longer methodologies, enables faster response to positives, and quicker remediation through sanitation. It also helps to prevent the transfer from zone to zone including transfer onto food contact surfaces of Zone 1.
Several such new methods are just coming to the market and will be discussed in future blogs.
What to do With the Test Results
A trend needs to be established, and after that, it should frequently be reviewed to identify trends (e.g., site that has positives with negatives due to intervention). Documented corrective action is required for any positive sample.
If there is a trend of increased frequency of pathogen detection by the swabs, it needs to be investigated to determine the reason and the action to be taken to reduce this frequency. Samples showing positive pathogen results should be followed up with additional investigations and root-cause analyses. Additionally, the cleaning and sanitation need to be intensified (“deep cleaning”).
If an area shows repeated positives, it is a potential harborage or problem area and will require attention until cleared. Corrective action must be implemented and documented. There must be a prearranged plan that will be implemented if a positive pathogen is detected. The plan should be zone specific. If a positive pathogen result is found in any zone, the area needs to be thoroughly examined both visually and through vector swabbing (see below) to determine the extent of the contamination and to ascertain potential causes of the problem.
Vector Swabbing
If a location results in a positive sample, vector swabbing is required. This means taking multiple environmental samples around the initial positive site. Vector sampling is usually done in a typical “star-burst” pattern around the initial positive site as shown in the figure below. Additional 10-15 swabs samples are taken around the initial positive sample.

Adopted from “Pathogen Environmental Monitoring program (PEM)”
Corrective Action
The specific corrective actions should be based on an evaluation of the likelihood of finished-product contamination based on the location of the initial positive site. A positive finding in Zones 2, 3 or 4 does not necessarily implicate finished product contamination. That decision should be made by the team and appropriate management personnel.
Corrective action should target the root-cause of the contamination. The food safety team should consider what changes (e.g., in personnel, training, equipment, process) will result in a permanent fix for the problem.
After the corrective action, additional testing may be necessary to verify the satisfactoriness of the corrective actions and to validate that the pathogen was eliminated from the area. All activities involving the corrective action should be documented.
