FDA proposes to restructure the Food Division by creating a single food safety and regulatory body under one leader

FDA recall of Abbott’s infant formula in the US
On February 17, 2022, the FDA first reported investigating infant illness related to products from Abbott Nutrition’s Sturgis, MI facility. Several months earlier (9/6/2021 to 12/18/2021), the FDA received complaints of infants that got ill after consuming powdered infant formula produced by Abbott Nutrition’s Sturgis, MI facility.
Three complaints reported infections due to Cronobacter sakazakii and one of Salmonella Newport infections. All four infants were hospitalized. Upon inspection of the Abbott facility, the FDA found several positive Cronobacter results from environmental samples. A recall was initiated by Abbott that included Similac, Alimentum, and EleCare, manufactured in the Sturgis facility. The products were distributed across the United States and exported to other countries. The recall resulted in a shortage of infant formula resulting in consumers seeking alternative brands or types of infant formula.

Infant formula recalled around the world
On the same day of the FDA recall notice, the CFIA announced their recall of the Abbott infant formula due to Cronobacter sakazakii and Salmonella in Canada. There have been no reported illnesses associated with consuming these products in Canada.
Since Abbott is a major international company, the recalled products were widely distributed worldwide. In addition to the US and Canada, the products were distributed to Australia, Bahrain, Barbados, Bermuda, Chile, China, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, Guam, Guatemala, Hong Kong, India, Indonesia, Israel, Jordan, Kuwait, Lebanon, Malaysia, Mexico, New Zealand, Oman, Peru, Puerto Rico, Qatar, Saudi Arabia, Singapore, South Africa, Sudan, Taiwan, Thailand, United Arab Emirates, United Kingdom, and Vietnam.
During the Sturgis facility inspection by FDA on September 2019, the FDA mentioned the lack of sufficient microbiological tests of infant formula finished products to meet the required microbiological quality.
Since several months have elapsed since the consumer complained until the recall, observers and the FDA have focused on how the recall process can become more effective.
Reagan-Udall Foundation scalding report

As a result of the infant formula debacle, Robert Califf (FDA commissioner) requested in July of 2022 that the Reagan-Udall Foundation1 convene an Independent Expert Panel to evaluate the FDA Human Foods Program comprehensively. Due to the situation’s urgency, the commissioner requested the evaluation be completed within 60 business days.
The report, issued in December 2022, was highly critical of the FDA. Some criticism includes a culture with no clear vision and mission; a disparate structure and a consensus governance model; competing priorities; and a lack of a robust and supportive leader and an ultimate decision-maker responsible for the Human Foods Program. Additionally, the FDA personnel are risk avert, operate in silos, are not encouraged to broaden their thinking outside of their division, and lack clarity of authority, resulting in frustration and substantial confusion among staff and leadership.
The Reagan-Udall Foundation Panel’s most significant recommendation was to create a new operating division within the HHS, with two commissioners reporting to the HHS secretary. The lack of a single identified person to lead was mentioned as one of the biggest deterrents to the program’s effectiveness.
Other recommendations that FDA leadership considered include:
Developing a clear and compelling vision, mission, and value statement.
Establishing a structure with a single leader to lead the food program.
Establishing regulatory decision-making rooted in scientific evidence.
Promoting top-quality staff and management.
The new vision of the FDA: reimaging of Human Foods Program
On January 31, 2023, Robert M Califf, MD, announced a new vision for the FDA Human Foods Program. He also announced a new role for the Office of Regulatory Affairs (ORA, the FDA’s field-based operations) in supporting the FDA organization as a whole.
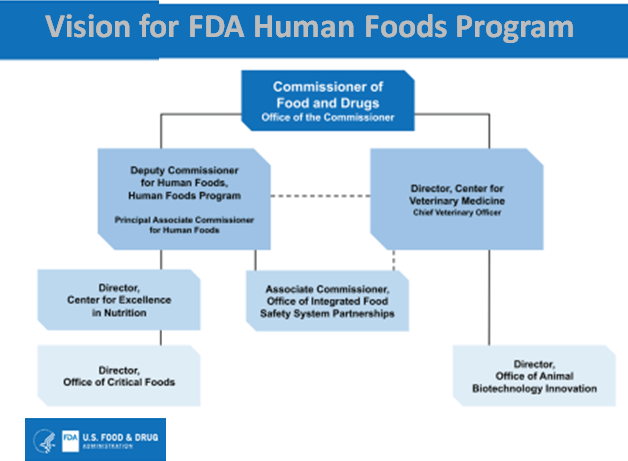
Dr. Califf believes that establishing a Human Foods Program led by a single leader reporting directly to the commissioner unifies and elevates the program. It will also remove redundancies, enabling the agency to oversee human food more effectively and efficiently. The new leader will provide strong, effective operational management of the program’s day-to-day operations. In addition, a team of executives for the program’s major areas of responsibility will be created to provide the necessary management infrastructure.
Under the FDA plan, the functions of the Center for Food Safety and Applied Nutrition (CFSAN), Office of Food Policy and Response (OFPR), and certain functions of ORA will bring together the newly envisioned organization called the Human Foods Program. A national search for a Deputy Commissioner for Human Foods overseeing the program will be initiated.
Other critical aspects of the proposed new Human Foods Program comprise of:
Creation of a Center for Excellence in Nutrition. The FDA proposes establishing an Office of Critical Foods within the center.
Establishment of an Office of Integrated Food Safety System This office will be a partnership focusing on elevating, coordinating, and integrating the food safety and response activities with state and local regulatory partners.
The proposal calls for establishing a Human Foods Advisory Committee to obtain independent expert advice on various issues. The committee will advise the FDA on emerging or challenging issues in food safety, nutrition, and innovative food technologies.
Another element is strengthening the information technology and analytical capabilities to fulfill the “New Era of Smarter Food Safety,” including support for the improvement in the workflow that will accompany these changes.
Reactions to the plan
Congresswoman Rosa DeLauro (D- CT) said, “Food safety is currently a second-class citizen at the Food and Drug Administration. No food policy experts are currently in charge of food safety at the FDA. The FDA’s deputy commissioner for food policy and response, Frank Yiannas, just announced his resignation in February. He complained that the decentralized structure of the FDA “significantly impaired the FDA’s ability to operate as an integrated food team and protect the public.”
Many stakeholders believe that the program’s success lies in finding the right leader to implement the new program. Stop Foodborne Illness CEO Mitzi Baum said that while many details are left unannounced, the group anticipates the restructuring “will facilitate accelerating decision-making, create seamless communication within a still fragmented agency, and forge consequential cultural changes.”
Finally, some object to Center for Veterinary Medicine (CVM) not being part of the new Human food division. Steven Mandernach, Executive Director of the Association of Food and Drug Officials (AFDO), in a statement given to New Food, said that he sees many positive aspects to the proposal by FDA Commissioner Robert M. Califf, MD. “In particular, we appreciate that his proposal elevates state and local programs that do much of the food safety work, creates a deputy commissioner with streamlined authority and focuses the agency’s nutrition mission in a Center of Excellence for Nutrition.” However, he also pointed out that there are still some gaps in the strategy announcement. “The other thing that I thought the commissioner still hasn’t fully understood is the relationship between the CVM and Human Food Supply. They are completely linked, and in addition, if you look at the animal and human food rules, they are nearly identical.” “Having them under separate chains with different reporting relationships just does not seem like a great thing for the industry.” “It was just one of those decisions that didn’t make much sense.” However, he said, “We look forward to collaborating with the Commissioner and others as details are developed to ensure the greatest opportunity for success.”
Consumer Reports stated that the FDA’s plans do not provide a direct line of authority over all human and animal foods program responsibilities. The International Dairy Foods Association (IDFA) is disappointed that the new Human Foods Program will not include the CVM, because there is a considerable overlap with human health, said Joseph Scimeca, senior vice president of regulatory and scientific affairs at the IDFA. He complained that the plan released does not show how to solve the bureaucratic, opaque process to update the hundreds of food and beverage standards that are outdated and hinder innovation.
Phyllis Entis (https://efoodalert.com/2023/02/05/sunday-supplement-proposed-new-fda-food-structure-leaves-pet-food-out-to-dry/) writes that the CVM is responsible for animal feed, pet food, and veterinary medicines was left behind in this reorganization. Entis suggests including the CVM in the new program and removing the word “human” from its title.
It is important to mention that the FDA plans to release additional details on its plan by the end of February. We will be waiting for more details.
