Biofilm and food safety: What is important to know?
Part 1: What are Biofilms?
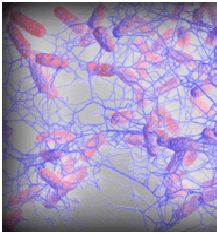 In nature, most bacteria do not exist as suspended (planktonic-free floating) cells. Bacteria live in a group (mass of bacterial cells) attached to each other and to surfaces, in a biofilm form.
In nature, most bacteria do not exist as suspended (planktonic-free floating) cells. Bacteria live in a group (mass of bacterial cells) attached to each other and to surfaces, in a biofilm form.
A biofilm is as a complex community of microorganisms, embedded in self-created extracellular polymeric substances (EPS). Therefore, the biofilm is a microbial population adherent to each other and to surfaces or interfaces enclosed in the matrix. In this complex biofilm network of EPS, the bacterial cells perform less as individual cells and more as a collective living system, frequently creating channels to deliver nutrients and water to the cells located inside the biofilm.
Bacteria create biofilm as a protection mechanism, for better survival in the environment. Cells in a biofilm are more resistant to cleaning and disinfection processes in the food industry. The bacteria in the biofilm attaches so firmly to the equipment’s surface that it becomes resistant to conventional sanitation procedures used by the food industry.
Various techniques such as molecular methods, chemical methods, and physical methods have been used to better understand the complex mechanism of biofilm formation, and to get an insight into how to create a process that will eliminate the biofilm formation and/or inactivate cells within the biofilm.
Moisture and nutrients from food (organic and inorganic material) are commonly found on production lines. These two elements bond together to create a conditioning layer. This layer allows for the initial attachment of the bacterial cells to the surface of the layer and the secretion of EPS. The production of ESP enhances the biofilm attachment to the food contact surfaces and protects the cells within the biofilm from the external stresses such as sanitizing agents.
How is Biofilm Formed?
The formation of biofilm can be described as a stepwise process, as shown in Figure 1, consisting of:
- Initial reversible attachment of the planktonic (free-floating) bacteria cell to the surface
- Irreversible attachment by the production of EPS
- Bacteria multiplication and development of biofilm structure
- Development of a microcolony covered by mature biofilm, stabilizing the microcolony from environmental stress
- Dispersion of cells from the biofilm into the surrounding, and the return of cells to their planktonic form.
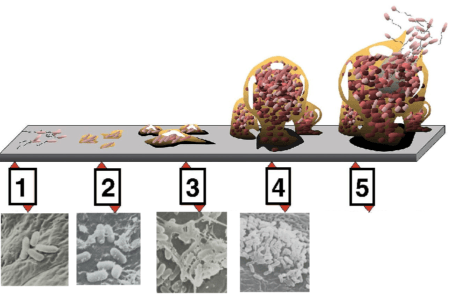 Figure 1: Formation of biofilm (adapted from Wikimedia , and Firstenberg-Eden et al )
Figure 1: Formation of biofilm (adapted from Wikimedia , and Firstenberg-Eden et al )
Organisms in a biofilm act less as individual cells and more as a combined living system and are significantly more resistant to environmental stresses such as antibiotics, sanitizers, chemical stress (pH, oxygen) and biocides than their planktonic cells. This increase in resistance to external stresses, as well as a shield against desiccation, is probably due to the presence of the EPS material.
Quorum sensing (cell to cell signaling) has been shown to play a role in biofilm formation, allowing bacteria to display a unified response that benefits the whole population. Research shows that transfer of antibiotic resistance gene is common in the biofilm environment. This transfer happens readily through conjugation or transformation.
Quorum sensing also enhances the ability of bacterial cells within the biofilm to access nutrients and increases their defense mechanism against competing bacteria and environmental stresses.
A recent publication in Cell showed that bacteria residing within biofilm communities could coordinate their behavior through cell-to-cell electrical signaling. Combining experimental data and mathematical modeling point to an extracellular potassium produced in the biofilm as a mechanism of changing the membrane potential of remote cells, thus, directing their motility.
Therefore, cells embedded within the biofilm can not only influence their behavior but influence the behavior of far-away cells through electrical signaling. A genetic mechanism appears to allow electrically mediated attraction between bacterial species
Why are Biofilms important to Food Safety?
Continual low-level contamination can be caused by biofilm, releasing bacteria, including pathogens and therefore, causing a food safety concern. Bacteria residing in biofilms are more resistant to antimicrobial agents and cleaning agents. Biofilms on processing equipment can reduce the lethality of the process.
Attached cell increased resistance to cleaning chemical because of the protection provided by the EPS layer. The decreased effectiveness of chemicals might also be as a result of cells being in a compact format reducing exposed surfaces that the chemical can make contact with the bacteria.
Product contamination can occur as bacteria detach from the microcolony periodically and can contaminate the processed foods and the lines. Pathogenic bacteria such as, Listeria, Salmonella, E. coli, or Pseudomonas, can form a multi-species biofilm, which is more stable and resistant to sanitizing agents.
Many outbreaks of foodborne disease are associated with biofilm. Research shows that biofilm has become a problem in food industries such as dairy, fish processing, poultry, meat, and Ready-To-Eat foods, because of the residing organisms increased resistance to external stresses.
Biofilm formation on food surfaces
Fruit and vegetable
Research has shown that bacteria can attach, colonize on the surfaces of plants, eventually forming biofilms. The ability of sanitizers to inactivate the bacterial cells in the biofilm is significantly reduced.
Disinfection steps by a diverse group of chemicals (e.g., chlorine, peroxide, surfactants, organic acids, etc.), UV, and irradiation were tested without successfully eliminating pathogens in the biofilms formed on fresh produce (without significantly affecting the product quality). Pathogens that are incorporated into mixed-species biofilms make them less susceptible to antimicrobial treatments as well as increase their tolerance to other stresses such as desiccation and UV.
Biofilms on plant surfaces are composed of a wide variety of bacterial species. The population dynamics of biofilms vary greatly corresponding to environmental conditions such as temperature, relative humidity, and the availability of nutrients.
Biofilm formation on plant surfaces is probably a survival mechanism for bacteria to endure harsh environment, including desiccation, UV exposure, and temperature fluctuations.
Research data (Bassam A. Annous 2005 ) shows Salmonella produces fimbriae and cellulose, starting biofilm formation, which helps the organism attach and colonize on melon and cantaloupe surfaces. Once attached to the fruit Salmonella cells survive better, and are less susceptible to the harsh sanitizing environment. The organism becomes difficult to remove from the cantaloupe surfaces due to attachment to inaccessible sites and biofilm formation on the cantaloupe rind surface, thus avoiding contact with the sanitizing solution.
After that, the surviving cells can be transferred from the surface of the fruit into the internal tissue of the fruit during processing, presenting a major obstacle for ensuring the microbiological safety of fresh-cut cantaloupe.
The produce most frequently associated with outbreaks include cantaloupe melons, apples (unpasteurized juice or cider), and leafy greens.
Meat
There are meat surfaces to which bacteria attach readily and other meat surfaces to which they attach much slower. The smooth chicken breast muscle (fascia) was the best surface for attachment of all bacteria examined. A linear relation between the concentration of bacteria attached to the surface and time during the attachment process was observed. On some surfaces, this linearity continued for a long time [teats of a cow, chicken breast with fascia, chicken skin]. Bacterial strain also impacts the attachment kinetics.
The bacteria are easily removed in the water film stage. With time, these bacteria could attach to the meat and become difficult to remove due to EPS formation. Brown et.al showed the attachment and biofilm formation by Campylobacter jejuni to extruded chicken meat. They demonstrated that chicken juice contributes to C. jejuni biofilm formation by covering and conditioning inert surfaces and is a source of nutrients. The organism preferentially attached to chicken juice particulates, increasing the attachment rate.
Escherichia coli O157:H7 from cattle had the ability to produce biofilm on food contact surfaces such as stainless steel. The attached organisms in the biofilm were able to transfer onto a variety of products such as raw meat, raw poultry, ready-to-eat deli meats, and produce products.
Strains isolated from cattle, retail chicken, and retail beef were able to form strong biofilms in addition to curli fimbriae and EPS production.
Spoilage and/or Pathogenic bacteria can attach to production food contact surfaces, and eventually form a biofilm. The biofilm formation is a major challenge to the meat industry due to the potential of cross-contamination of the meat, causing short-shelf life and/or spread of diseases.
Equipment
Both Gram negative and Gram positive bacteria present on food processing equipment can colonize on stainless steel. Gram-negative bacteria produced much greater biofilms on stainless steel than Gram positives.
The ability of Listeria monocytogenes to develop biofilms and survive on different types of materials was studied by Wong 2002. The materials studied included two types of stainless steel (304 and 316L), two types of rubber (Buna-N and silicone), and three materials used in conveyor systems (Polyester 3000 and TURE-2 used as belting material, and Delrin, a hard plastic, used in rollers for conveyor belts).
Biofilm formation was best supported by the plastic material Delrin, followed by stainless steel type 304. Food grade silicone rubber and stainless steel type 316L surfaces were the most resistant to biofilm development. L. monocytogenes was capable of forming a biofilm at 10°C, in low nutrient medium on all surfaces tested. The 5-day biofilm cells were more resistant to cleaning and sanitizers as compared to the 2-day biofilms.
The persistence of bacterial cells within a biofilm in the whole food industries including cheeses, dairy products, raw foods, and ready to eat produce continue to contribute to the short shelf-life and/or human pathogenic foodborne outbreak. ESP Material produced by the bacterial cells in biofilms and the complexity of processing equipment makes it difficult to remove and/or inactivate these bacterial cells on food processing surfaces. Therefore, biofilm control relies on the implementation of effective cleaning and sanitizing procedures. Also, the design of processing equipment and the food processing environment that reduces and/or eliminates the accumulation of bacterial and that allows easy, and thorough soil removal can be a significant issue in controlling biofilm formation.
Comming soon our second chapter about Part 2: What are the best control strategies?
