Artificial tear eye drops caused an outbreak of extensively drug-resistant Pseudomonas aeruginosa
The outbreak
The CDC, FDA, and state and local health departments are investigating a multistate outbreak of a highly drug-resistant strain of Pseudomonas aeruginosa. According to the CDC, the outbreak was caused by artificial tears. The Pseudomonas aeruginosa strain is carbapenem-resistant. It is Verona integron-mediated metallo-β-lactamase and Guiana extended-spectrum-β-lactamase (VIM-GES-CRPA). Such a strain was never before been reported in the US. The CDC reports that while the outbreak is associated with multiple types of infections, the most notable is an eye infection. Isolates have also been isolated from cultures of sputum or bronchial wash (15), cornea (17), urine (10), other nonsterile sources (4), and blood (2), and from rectal swabs (26).
Victims of the outbreak
On March 14, 2023, the CDC reported that 68 patients in 16 states (CA, CO, CT, FL, IL, NC, NJ, NM, NY, NV, PA, SD, TX, UT, WA, and WI) were infected by the rare strain of P. aeruginosa that is extensively drug-resistant. A cluster of 37 patients was linked to four healthcare facilities. The outbreak caused three deaths. Eight patients had vision loss, and four had surgical removal of the eyeballs.
The product
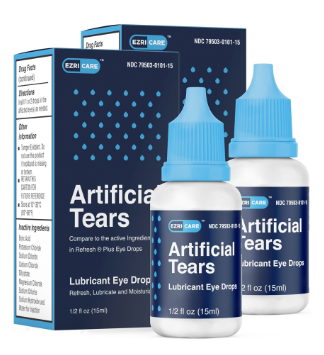
CDC observed that most patients in the outbreak reported using artificial tears. While over ten different brands of artificial tears were reported, EzriCare Artificial Tears, a preservative-free, over-the-counter product packaged in multidose bottles, was the brand most frequently reported.
EzriCare was the common artificial tears eye drop product identified across the four healthcare facility clusters. Testing of an opened bottle of EzriCare from patients with and without eye infections identified the presence of VIM-GES-CRPA Pseudomonas aeruginosa strain in multiple lots. In February, the FDA warned consumers not to purchase or use EzriCare Artificial Tears due to potential contamination and not to use Delsam Pharma’s Artificial Eye Ointment due to potential bacterial contamination. EzriCare parent company (India-based Global Pharma Healthcare) failed to provide microbial test results for its OTC products, including EzriCare and Delsam Pharma’s Artificial Eye Ointment.
The FDA cited Global Pharma Healthcare for failing to provide a product with adequate temper evidence and distributing drugs without appropriate preservatives.
In February, two additional products Apotex’s Brimonidine Tartrate Ophthalmic Solution, 0.15%, and Pharmedica USA, recalled their eye drops. However, these eye drops have not been linked to the current infection.
Two other products — Pharmedica’s USA Purely Soothing, 15% MSM Drops, and Apotex’s Brimonidine Tartrate Ophthalmic Solution, 0.15% — were also pulled by their makers, though they are not related to the outbreak.
According to The Miami Herald Teva Pharmaceuticals has recalled ~716,000 bottles of Clear Eyes, Once Daily, Eye Allergy itch relief, eye drops for failed impurity testing.
The organism
The Pseudomonas aeruginosa VIM-GES-CRPA isolates associated with this outbreak were resistant to cefepime, ceftazidime, aztreonam, piperacillin-tazobactam, aztreonam, carbapenems, ceftazidime-avibactam, ceftolozane-tazobactam, fluoroquinolones, polymyxins, amikacin, gentamicin, and tobramycin. Before this outbreak, no strain of Pseudomonas aeruginosa had this combination of multi-drug resistance genes.
Due to the extensive drug resistance of this strain, the CDC said bacteriophages with activity against the strain could be a potential treatment option. The University of California (San Diego’s Center for Innovative Phage Applications and Therapeutics) is investigating this option.
