Another FSMA Rule Takes Effect: Foreign Supplier Verification Program (FSVP)
 The FDA FSMA rule on Foreign Supplier Verification Program (FSVP) for Importers of Food for Humans and Animals is final, and the first compliance date begins on May 30, 2017.
The FDA FSMA rule on Foreign Supplier Verification Program (FSVP) for Importers of Food for Humans and Animals is final, and the first compliance date begins on May 30, 2017.
This rule imposes far reaching changes on food importers and requires review of the food safety practices of foreign suppliers and their compliance history. Foreign suppliers must be approved in advance and must have a written program before they can be a supplier in the US. The rule also requires new information to be submitted for customs entries: the FSVP importer must now be declared for each importation of FDA-regulated food (including dietary supplements) unless exempt.
The goal of the program is to protect US consumers from potentially contaminated or dangerous imported foods. This is carried out by requiring importers to ensure that imported foods are produced in a way that matches or equals the US FDA standards for food safety and preventative control.
Program Scope
FSVP is a program that must be put into place by importers of human and animal food, to verify that their foreign suppliers are producing food in a manner that provides the same level of public health protection as products produced domestically, and ensuring that the supplier’s food is not adulterated and not misbranded with respect to allergen labeling.
The importers are responsible for determining the hazard associated with each food, and evaluate the risk it poses. They need to conduct supplier identification activities, and take corrective action if needed. They must ensure that they import foods only from approved suppliers, based upon the risk posed by the imported food and the supplier’s performance. Each supplier requires to catty out a separate FSVP for each food.
If the importer obtains a certain food from a few different suppliers, a separate FSVP will be required for each of those suppliers. Similarly, if the importer obtains many different foods from a single supplier, a separate FSVP will be required for each food.
Hazard Analysis
An importer is required to determine if there are any hazards that require control such as biological hazards from parasites and disease-causing bacteria; chemical hazards such as radiological hazards, pesticide and drug residues, natural toxins, food decomposition, unapproved food or color additives, and food allergens; or Physical hazards, such as glass or metal. This analysis must evaluate the probability of their occurrence in absence of controls, and the severity of injury or illness that might result.
The analysis considers factors such as: Formulation of the food, establishment design, raw materials used, transportation methods, sanitation and employees’ hygiene, packaging and labeling activities, storage and distribution.
Food Risk and supplier Performance
An importer must evaluate, in addition to the hazard analysis, the suppliers’ procedures, processes, and practices related to food safety. He must also assess the suppliers’ adherence to FDA food safety regulations, and their food safety history including corrective action.
Supplier Verification
Written procedures must be established by the importer that the imported products are only from approved foreign suppliers and must establish supplier verification activities. This might include annual audits of the supplier’s facility, sampling and testing, and review of the supplier’s food safety records.
Corrective Action
Importers are required to take appropriate corrective actions if they determine that a foreign supplier has not used processes and procedures that provide the same level of public health protection as required, or that the supplier produces food that is adulterated or misbranded with respect to allergen labeling.
Included Categories
Compliance is required by May 30 for the following foods/ supplier categories:
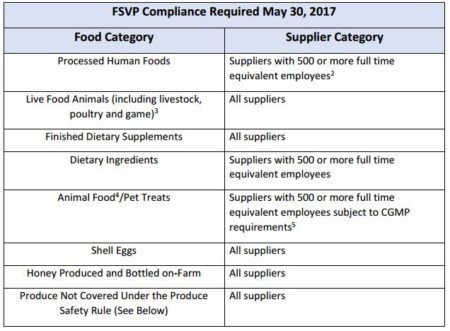

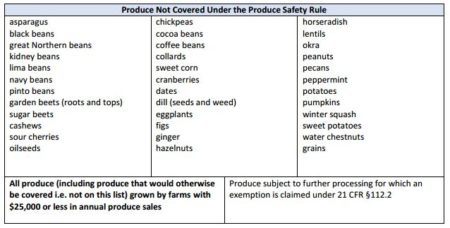
Exemptions
Some foods are exempt from these regulations. For example: meat, poultry and egg products subject to USDA regulations; alcoholic beverages; food imported for research or evaluation, provided it is not for retail sale, is properly labeled and is accompanied by an electronic declaration at entry; etc.
Unique Facility Identifier
A key to this regulation is the creation of Unique Facility Identifier (UFI). The Importer must provide its name, electronic mail address, and UFI recognized as acceptable by the FDA for each line entry of food product offered for importation into the United States.
On March 31, 2017, The FDA issued guidance recognizing the DUNS number as an acceptable UFI for the FSVP regulation.
The FDA details the entry into the United States (U.S.), the U.S. Customs and Border Protection (CBP) Automated Commercial Environment (ACE) systems that are in place.
On May 11, the FDA updated the regulations, by clarifying the categories of imports that must comply by May 30. The importers that must comply need to provide specific identification for each line entry of food product offered for importation into the United States. The importer identification requirement includes the submission of the FSVP importer’s name, e-mail address, and UFI recognized as acceptable by FDA. Foreign persons cannot serve as the FSVP importer.
