A simple new blood test for accurately distinguishing between bacterial and viral Infection in 15 minutes
 Worldwide antibiotic abuse is a significant public health and economic concern. Abuse of use of antibiotics is done by both overuse and underuse. Overuse is performed when treating a non-bacterial disease, such as a viral infection. Bacterial and viral infections are often indistinguishable. Antibiotics are, in many cases, erroneously prescribed for viral infections. One of the most frightening consequences of antibiotic overuse is the emergence and spread of antibiotic-resistant bacteria.
Worldwide antibiotic abuse is a significant public health and economic concern. Abuse of use of antibiotics is done by both overuse and underuse. Overuse is performed when treating a non-bacterial disease, such as a viral infection. Bacterial and viral infections are often indistinguishable. Antibiotics are, in many cases, erroneously prescribed for viral infections. One of the most frightening consequences of antibiotic overuse is the emergence and spread of antibiotic-resistant bacteria.
Antibiotic underuse could cause prolonged disease duration and an increased rate of disease-related complications, both of which may be avoided with prompt treatment of the bacterial Infection.
The US Centers for Disease Control and Prevention (CDC) estimates that over 80 million antibiotic prescriptions are given in the US annually, in outpatient settings, to treat viral infections, for which they are ineffective and inappropriate.
Therefore, it is crucial to have a technology that can rapidly and accurately distinguish between bacterial and viral infections. Such technology can reduce the overuse of antibiotics in viral infections.
MeMed technology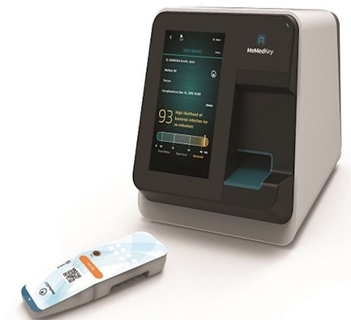
MeMed Diagnostics (https://www.me-med.com/) is a personalized diagnostics company founded in 2009, with headquarters in Tirat Carmel ,Israel and an office in Cambridge, MA. They developed an immunoassay test to differentiate bacteria from viruses using chemiluminescence detection technology.
While most infectious disease tests look for a specific pathogen, MeMed BV is an advanced host immune response test that measures the levels of immune system proteins and applies proprietary algorithms to generate an immune signature.
The devices distinguish between bacterial and viral infections based on patients’ immune responses to different infection types, enabling physicians to get rapid (15 minutes) and actionable clinical information to make informed antibiotic treatment decisions.
The MeMed Key cartridges allow simultaneous measurements of up to four different analytes. The MeMed assay separates bacteria from viruses by the analysis of three host response proteins – TRAIL, IP-10, and CRP. The 3 proteins are found at very different levels in a patient’s blood depending on if the immune system is fighting a virus or bacteria. The system computationally integrates the levels of the 3 proteins.
The system can be used in the hospital laboratory, emergency departments upon hospital admission, urgent care centers, or doctor’s offices. The MeMed system is claimed to be accurate and easy to use, making it possible to conduct highly sensitive, rapid, multiplexed protein measurements that previously could only be done on expensive central lab equipment.
FDA granted 510K clearances

In the September of 2021, the MeMed assay was granted a 510K clearance from the FDA. The FDA clearance was based on a multi-center blinded clinical validation study enrolling over 1,000 children and adults. The technology has been cleared by the FDA for both children and adults. The test is for point-of-care at runs on the MeMed Key® platform.
The company was also granted CE clearance in 2020, allowing it to market its COVID-19 Severity test in Europe and the United Kingdom.
Articles substantiating the technology
The clinical trial was carried out to substantiate the 510K encompassed thousands of patients and was published ( in Journal of Infection (A systematic review of host biomarkers B. Leticia Fernandez-Carballoa,∗ , Camille Escadafal a, Emily MacLeanb, Anokhi J. Kapasi a, Sabine Dittricha, 2021, Journal of Infection 82,1-10; https://www.journalofinfection.com/action/showPdf?pii=S0163-4453%2821%2900085-2).
In the study, four biomarker entries tested in blood samples had sensitivity/specificity of more than 0.90/0.80. Only 12 additional biomarkers were identified with sensitivity/specificity of more than 0.65/0.65. Protein biomarkers with the highest reported performances include a combined biomarker signature (CRP, IP-10, and TRAIL) and human neutrophil lipocalin (HNL).
A study, funded by the EU commission and published in Clinical Microbiology and Infection (Papan C et al., A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: a prospective, multicentre cohort study, Clinical Microbiology, and Infection, https://doi.org/ 10.1016/j.cmi.2021.10.019) was used for the CE certification.
The MeMed’s technology was validated in a broad pediatric group (n=1,008; >90 days to 18 years). The data demonstrated the test’s potential to improve antibiotic stewardship in children with fever, without source and respiratory tract infections, at emergency departments and wards in Italy and Germany. The trial results showed 94% sensitivity, 94% specificity, 73% PPV, and 99% NPV. The results validate the high diagnostic performance of the TRAIL/IP-10/CRP utilization in a broad pediatric cohort and support its potential to reduce antibiotic overuse in children with viral infections.
