A new report: FSIS Foodborne Illness Outbreak Investigations, Fiscal Year 2021
A report published by FSIS (https://www.fsis.usda.gov/sites/default/files/media_file/2022-06/fy21-fsis-outbreak-investigations-annual-report.pdf)

The United States Department of Agriculture’s Food Safety and Inspection Service (FSIS), Office of Public Health Science, Applied Epidemiology Staff, coordinates the FSIS response to foodborne illness outbreaks that may involve FSIS-regulated products. This includes outbreaks that involve four foodborne pathogens that most frequently affect FSIS-regulated products: Salmonella, Shiga toxin-producing Escherichia coli (STEC), Listeria monocytogenes (Lm), and Campylobacter. FSIS may investigate illnesses associated with other less common foodborne pathogens (e.g., Clostridium botulinum) if they are associated with FSIS-regulated products. In coordination with our public health partners (the Centers for Disease Control and Prevention (CDC) and state public health and agriculture departments), FSIS collects and evaluates epidemiologic, laboratory, and traceback information to determine if there is an association between an FSIS-regulated product and human illnesses. Epidemiologic information can include details like which foods ill people ate, where they purchased these foods, and where they live. Laboratory information can include comparing bacteria from FSIS collected product samples and ill people to see if they are genetically similar or have similar characteristics. Traceback activities may include determining where the product was sold (e.g., grocery store, deli counter, or restaurant) or the source of a product (e.g., the federally inspected slaughter or processing facility). Depending on the evidence collected during an investigation, FSIS may have enough detailed exposure and product information to take one or more actions to prevent additional illnesses. This report summarizes outbreaks that FSIS investigated from October 1, 2020, to September 30, 2021, the Fiscal Year 2021 (FY 2021). This report also highlights critical after-action reviews conducted and published in FY 2021. While the number of outbreaks FSIS investigates fluctuates annually, the COVID-19 pandemic likely decreased the number of reported foodborne illness outbreaks in FY 2021. Several factors likely played a part in this decrease, including healthcare-seeking behaviors, hygiene practices, and where consumers ate their meals. For the previous three fiscal years, the outbreaks investigated by FSIS are as follows: 16 in 2018, 16 in 2019, and 12 in 2020.
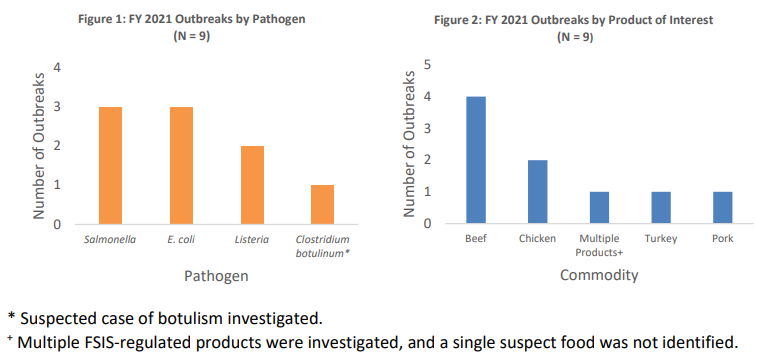
During FY 2021, FSIS investigated nine outbreaks in coordination with local, state, and federal public health partners. These outbreaks involved approximately 200 illnesses and 60 hospitalizations. CDC notified FSIS about most of these outbreaks. Seven (77.7%) outbreaks involved illnesses in more than one state. Of the nine outbreaks investigated by FSIS in FY 2021, Salmonella and STEC were each implicated in three outbreaks, with Lm implicated in two. The ninth investigation involved a suspected case of botulism, but neither the botulinum toxin nor the Clostridium botulinum microorganism was detected in the suspect product (Figure 1). The Salmonella outbreaks involved four serotypes: Hadar, Enteritidis, Typhimurium, and Infantis, with both Typhimurium and Infantis in one outbreak. Serogroups O157:H7 and O145 caused STEC outbreaks. Beef was the most common food product linked to outbreaks in FY2021(Figure 2).
FSIS may ask an establishment to recall product from commerce to protect public health when it is found to be associated with an outbreak. A recall is a firm’s removal of distributed meat or poultry products from commerce when there is a reason to believe that such products are adulterated or misbranded under the provisions of the Federal Meat Inspection Act, the Poultry Products Inspection Act, or Egg Products Inspection Act. FSIS may issue a Public Health Alert (PHA) when the Agency determines that meat, poultry, or egg product may be associated with human illness and no adulterated product remains in commerce, or FSIS cannot determine what specific regulated product is implicated by the illnesses and thus adulterated. In FY 2021, outbreak investigations led to three recalls of FSIS-regulated products and two PHAs.
Assessment of outbreaks associated with FSIS-regulated products is crucial to FSIS’ mission to prevent foodborne illness and protect public health. FSIS routinely conducts after action reviews (AAR) after foodborne outbreak investigations to identify lessons learned that can help improve response and prevent future illnesses. Applying and sharing learned outbreak lessons may lead to improved food safety policies and strengthen collaborative investigations with public health partners. FSIS conducted AARs for several FY 2021 outbreak investigations to identify best practices and areas for improvement. Below are highlights from AARs that were conducted during FY 2021. FSIS conducted an AAR for an FY 2021 outbreak of Salmonella Enteritidis associated with raw, frozen, breaded, and stuffed chicken products. During this outbreak, investigators noted that consumers used various cooking methods included on the manufacturer’s cooking instructions label (oven) and methods that were not included on the manufacturer’s label (such as air fryers and microwaves). • Establishments that produce raw, frozen, breaded, and stuffed chicken products are encouraged to review FSIS Labeling Policy Guidance Uncooked, Breaded, Boneless Poultry Products. • FSIS brought concerns about these products to the National Advisory Committee on Meat and Poultry Inspection (NACMPI) to request advice on reducing illness. NACMPI made recommendations below for agency consideration: o FSIS review of labels and cooking instructions. o FSIS review of establishment food safety programs. o FSIS conducts exploratory sampling for Salmonella in producing establishments. FSIS conducted an AAR for an FY 2021 outbreak of Listeria monocytogenes associated with ready-to-eat chicken. • Whole genome sequencing of isolates generated by FSIS routine sampling provided investigators with crucial data, highlighting the importance of these analyses to generate hypotheses related to possible food sources and inform outbreak investigations. • Establishments that produce post-lethality exposed (i.e., products that are exposed to the environment after the lethality step such as cooking that destroys bacteria present in the raw product is complete) ready-to-eat products are encouraged to review FSIS compliance guidelines for controlling Lm. • Rapid coordination and communication allowed for data sharing between FSIS, CDC, state public health partners, and industry and facilitated the rapid response to this outbreak. FSIS was able to obtain exposure information quickly and rapidly collect and conduct traceback activities of the product for a complicated outbreak.
