A New Method for the Detection of Salmonella in Powdered Dairy Products
 The Journal of Dairy Sciences reports that a team of researchers from China (Zhao et al. J. Dairy Sci. 100:3480–3496 May 2107) developed a new method for the detection of Salmonella in infant powdered milk.
The Journal of Dairy Sciences reports that a team of researchers from China (Zhao et al. J. Dairy Sci. 100:3480–3496 May 2107) developed a new method for the detection of Salmonella in infant powdered milk.
The developed method is claimed to be rapid, specific, and sensitive. It is is based upon loop-mediated isothermal amplification technique combined with a lateral flow dipstick (LAMP-LFD) as the detection step.
Loop-Mediated Isothermal Amplification Technique (LAMP)
LAMP is a powerful new nucleic acid amplification method that detects very low levels of DNA. The method amplifies a few copies of target DNA with high specificity, efficiency and rapidity. The method uses a set of 4 specifically designed primers that recognize 6 distinct sequences of target DNA, and a DNA polymerase.
The cycling reaction can result in the accumulation of 109 fold of copies in less than 1 hour. The method is claimed to be more specific and less susceptible to interference than PCR, it is very fast, without the need of denaturing step.
Target Genes
The target gene invA encodes a Salmonella invasion protein and is thus considered a virulence gene located on Salmonella pathogenicity island (SPI), and is used frequently for the detection of Salmonella. The SPI4 region includes genes from siiA to siiF that are important for adhesion to polarized epithelial cells, and plays an important role in Salmonella pathogenicity.
The authors claim that this is the first attempt to use LAMP and the siiA gene to detect Salmonella.
Lateral Flow Dipstick (LFD)
Lateral flow immunoassays dipsticks are used routinely to detect pathogens in food. Lateral flow dipstick use a sandwich type ELISA and the majority use polyclonal antibody as a capture antibody and a monoclonal antibody as the detection antibody. The antibodies are fixed on a hydrophobic membrane in immobilized in lines. Their role is to react with the analyte bound to the conjugated antibody. Recognition of the sample analyte results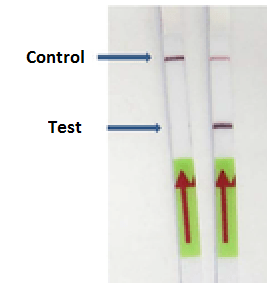 in an appropriate response on the test line, while a response on the control line indicates the proper liquid flow through the strip.
in an appropriate response on the test line, while a response on the control line indicates the proper liquid flow through the strip.
In the LAMP-LFD assay LFD strip is inserted into a tube that allows the strip to be immersed in the amplified sample. The sample migrates through theconjugate pad, which contains antibodies specific to the target analyte and are conjugated to colloidal gold and latex microspheres. The sample, together with the conjugated antibody bound to the target analyte, migrates along the strip into the detection zone.
A number of researchers have combined LAMP with LFD. In this combination the LFD is soaked in LAMP amplified sample and the liquid travels by capillary action across the membrane to react with the antibodies and provide a color band.
Elimination of carryover Contamination
The high sensitivity of LAMP can become its largest potential disadvantage because trace left over material can be amplified and detected, causing false positive results, after several times of detection in the same place. Therefore, there is a need to eliminate any contamination from previous LAMP reactions.
To reduce incidence of LAMP contamination, the authors applied propidium monoazide (PMA) to eliminate carryover contamination of LAMP. The appropriate concentration of PMA diluted in water was applied to the working environment of any contaminated area and adequate light exposure conditions were used to complete the decontamination process.
Results
A very specific and conserved Salmonella target gene siiA was used to establish the LAMP-LFD detection method for Salmonella in powdered Infant formula.
In this study, the limit of detection of the LAMP-LFD for inoculated powdered infant formula, without enrichment was 2.2 cfu/g, which is 100x lower than the limit of detection for most PCR methods. A pure culture study of 21 Salmonellastrains (with limited number of serotypes), and 60 inoculated samples of powdered infant formula yielded all positive results. 31 non-Salmonella strains (75% gram positive), including 20 non inoculated samples all yielded negative results.
While more testing of this method is required, the reported method seems to be very rapid, specific, and sensitive for the detection of Salmonella in powdered infant formula. PMA needs to be used to eliminate the LAMP carryover contamination.
