A new antibiotic Fabimycin may be effective against 200+ antibiotic-resistant gram-negative bacteria
Crisis of antibiotic resistance

Many deadly bacteria are becoming resistant to antibiotics meant to fight them. The rise of these superbugs creates a crisis of antibiotic resistance. Antibiotic resistance threatens modern medicine and the ability to perform surgeries and complicated organ transplants safely. Drug resistant bacteria are not responding to first or even second-line antimicrobial treatments.
In 2017, the WHO identified a list of bacteria that present a significant threat to human health and require new antibiotics. The list included bacteria that are in critical need such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae. A majority of the pathogens listed by the WHO are Gram-negative bacteria.
Bacterial resistance to antibiotics is a critical global public health threat, killing at least 1.27 million people worldwide and associated with nearly 5 million deaths in 2019. In the U.S., more than 2.8 million antimicrobial-resistant infections occur each year. According to the CDC’s report, more than 35,000 people die as a result.
The world is facing a problem where new drugs are needed since resistance eventually happens to all drugs. WHO concludes that one of the biggest threats to global health and food security is the lack of new antibiotics.
Reasons for bacterial resistance to antibiotics
The most apparent cause of antibiotic resistance is the misuse and overuse of these drugs. Research has shown that doctors prescribing the wrong antibiotics or mistakenly prescribe antibiotics when not needed, contributed to the current health crisis. Studies have shown that many times doctors prescribe to people in intensive care units antibiotics that might not be necessary.
Another source of antibiotic resistance is farm animals that consume antibiotics. Of all antibiotics sold in the US, approximately 80% are sold for use in animal agriculture. Farmers are using high rates of antibiotics in animals to boost growth rates and prevent infections.
Suggested approaches to solve the antibiotic resistance problem
Pew reported on new antibiotic development as of December 2020. One must remember that developing new drugs involves lots of time, effort, scientific research, and expense. Only 1 in 5 drugs that get to the initial testing phase in humans will receive approval from the FDA. The economics of such development is challenging because only a small number of patients need these drugs.
Pew estimates that the pipeline shows 43 new antibiotics in development. Fifteen were in Phase 1 clinical trials, 13 in Phase 2, 13 in Phase 3, and two have had new drug applications submitted.
At least 19 antibiotics in clinical development can potentially treat infections caused by ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp). Roughly 1 in 4 drugs in the pipeline represent a novel drug class or mechanism of action. None of these are potentially active against Gram-negative ESKAPE pathogens or WHO critical threat pathogens.
Alternative nonantibiotic approaches to treat infections, including bacteriophages, vaccines, and antibodies, are being tested. Compounds from plant-based sources, such as green tea, Persian shallots, and turmeric, are also being considered.
A new antibiotic developed: Fabimycin
The compound
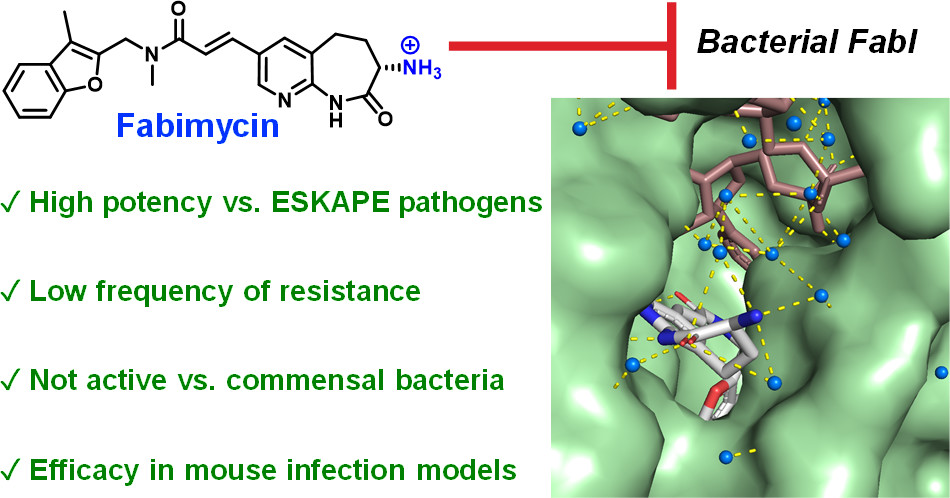
Dr. Hergenrother, professor of chemistry (U Illinois), and his team created an antibiotic that would successfully accumulate in Gram-negative cells. The work was published in Parker et al. “An Iterative Approach Guides Discovery of the FabI Inhibitor Fabimycin, a Late-Stage Antibiotic Candidate with In Vivo Efficacy against Drug-Resistant Gram-Negative Infections,” ACS Cent. Sci. 2022.
The research targeted the FabI enzyme, responsible for catalyzing the rate-determining step in bacterial fatty acid biosynthesis. The solid outer membrane and promiscuous efflux pumps of these pathogens prevent many antibiotics from reaching these targets. However, the enzyme FabI catalyzed the rate-determining step in bacterial fatty acid biosynthesis and was a great candidate. The molecule chosen by the researchers, Fabimycin, showed superior potency in initial tests.
Clinical isolate testing
The antibacterial activity of fabimycin was tested against a panel of 200+ multidrug-resistant Gram-negative clinical isolates. Fabimycin has impressive activity against Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. The compound showed low MICs for the clinical isolates. Fabimycin demonstrated high specificity for pathogenic bacteria versus normal microflora. This finding suggests that fabimycin could be less damaging to gut microflora than typical broad-spectrum antibiotics.
Efficacy in mouse models
 Using the effective dose of fabimycin in mice, the researchers evaluated the efficacy of fabimycin in mice infected with a challenging, drug-resistant strain of Escherichia coli. Fabimycin was administered intravenously three times a day. The treatment reduced drug-resistant bacteria in mice’s spleen, bladder, liver, and kidney tissues to pre-infection levels or below.
Using the effective dose of fabimycin in mice, the researchers evaluated the efficacy of fabimycin in mice infected with a challenging, drug-resistant strain of Escherichia coli. Fabimycin was administered intravenously three times a day. The treatment reduced drug-resistant bacteria in mice’s spleen, bladder, liver, and kidney tissues to pre-infection levels or below.
Current status
The advancement of this class of FabI inhibitors has been complicated by the uniquely poor stability of these compounds in mouse plasma. The researcher believe that fabimycin efficacy may improve as it is used to treat infections in humans because of (i) the encouraging activity of fabimycin in mouse infection models and (ii) due to fabimycin being dramatically more stable in rat and human plasma.
A clinical trial in humans is the next step.
