Rapid Methods for Food Pathogens- an Update
 The USDA / FSIS recently updated the list of Foodborne Pathogen Test Kits Validated by Independent Organizations.
The USDA / FSIS recently updated the list of Foodborne Pathogen Test Kits Validated by Independent Organizations.
The rapid detection of pathogens in food is important to ensure the safety of consumers. Traditional methods to detect foodborne pathogens rely on time-consuming growth in culture media, followed by isolation, biochemical identification, and sometimes serology.
Recent advances in technology result in faster detection and identification of pathogens, more convenient, more sensitive, and more specific than conventional methods. These new methods are often referred to as “rapid methods”.
It is important to remember that in most foods, rapid methods still lack sufficient sensitivity and specificity for direct testing of the food; therefore, the foods still need to be enriched in a culture media before analysis.
In the USDA / FSIS document there are 3 separate lists of methods: for Salmonella, Campylobacter, and Listeria.
The list for Salmonella includes more than 25 companies, many of which offering multiple assay options, resulting in more than 40 methods listed.
Methods
The methods include antibody-based assays, genetic amplification methods and newer sensor development methods.
Growth based methods: Including unique enrichment methods, chromogenic plates, and film based methods.
Immunological-Based Methods: Include enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay. Also included are immune separation methods such as immunomagnetic separation (IMS).
Molecular Detection Methods: (nucleic acid-based methods): include simple polymerase chain reaction (PCR), multiplex PCR, real-time PCR (qPCR), nucleic acid sequence-based amplification (NASBA), loop-mediated isothermal amplification (LAMP) and oligonucleotide DNA microarray.
Biosensor devices: A Biosensor is an analytical device that consists of two main elements: a bioreceptor and a transducer. The bioreceptor is responsible for recognizing the target analyte and can either be a biological material (enzymes, antibodies, nucleic acids and cell receptors), or biologically derived material, or Biomimic: imprinted polymers and synthetic catalysts. The transducer that converts the biological interactions into a measurable electrical signal can be optical, electrochemical, mass-based, thermometric, micromechanical or magnetic.
Validation
The methods were validated by recognized independent organizations (e.g., AOAC, AOAC-RI, AFNOR, MicroVal, NordVal). None of the test kits listed is implicitly approved by USDA FSIS. A validated test kit must also be fit for purpose (i.e. validated for the appropriate matrix and sample size) and appropriate for the specific application in a food safety program.
In 2011 FDA issued guidelines for validation (FDA Methods Validation Guidelines for Microbial Pathogens) . It states that for commercially-available microbiological diagnostic kits:
- Performance parameters must be fully validated in a collaborative study by an independent accrediting body e.g. AOAC-OMA, AFNOR, etc.
- Each lab must perform an in-house verification for the “first use” and for subsequent use of the method;
- Lab controls shall be used per lot to re-verify the method.
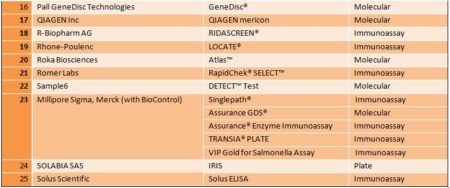
List of methods
The USDA / FSIS list is organized by organism and includes the manufacturer, assay commercial name, the method of external validation, the sample size, and the matrices. From the USDA / FSIS list the following table was created: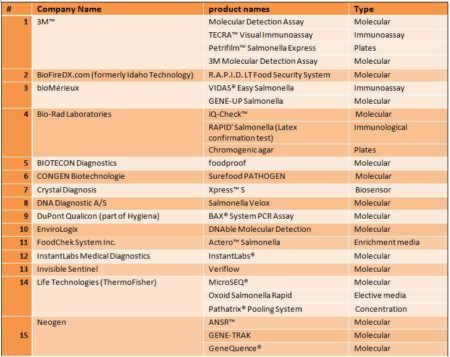
Status of Rapid Methods
Rapid methods have not been adopted as rapidly as projected when they were first introduced. While the new methods offer advantages in technology, speed and accuracy, they represent an incremental progress, rather than a revolution.
With the large number of competitors in the pathogen system arena, it is hard to differentiate among the technologies and find the clear winners. Every small progress of one technology is soon followed by a competitor that makes it slightly better.
With 40 different assays on the market it is increasingly more difficult for companies to choose a new rapid method. It is becoming increasingly more difficult to decide to invest in a new rapid method. This might be one of the reasons that more companies are moving to third party testing laboratories (http://www.strategic-consult.com/2014/11/growth-food-safety-testing-gone/). Small and mid-sized food plant labs are closing, and looking to third-party contract testing labs for their pathogen testing.
The extensive validation requirement as part of FSMA, and the increased training and documentation requirement also drive smaller manufacturers toward contract laboratories.
