fasdf@sadf.tu
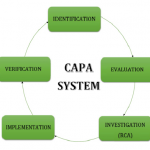
What is CAPA?
CORRECTIVE ACTION & PREVENTIVE ACTION What are Corrective Action and Preventive Action? Corrective action is an action taken to prevent the re-occurrence, Whereas Preventive action is an action to prevent the occurrence. FDA investigators focus on CAPA during inspections because it is a roadmap to identify potential and existing problems at a company. CAPA documentation provides FDA investigators, auditors, and executive management a means to review problems. Therefore, effective management of the CAPA system is critical to compliance. By following five essential steps, companies can be CAPA compliant and ensure a successful audit or inspection. The failure to either have a CAPA process,...
fasdf@sadf.tu
Why are there More Recalls Due to Listeria monocytogenes
Why are there More Recalls Due to Listeria monocytogenes In the past few months there have been a number of recalls due to L. monocytogenes. Just in the month of September there were four recalls. The question is why are we seeing this increase in recalls? Is it a sign of more problems or a sign of the industry getting better in catching the problems earlier? Let’s review the following cases: Blue Bell Ice cream September 21: FDA announced that the company is voluntarily recalling some half-gallon and pint-sized chocolate chip cookie dough and half-gallon “Cookie Two Step” products because they were made...
fasdf@sadf.tu
Kellogg recalls Eggo® Nutri-Grain® Whole Wheat Waffles due to Fear of Listeria
Eggo® Nutri-Grain® Whole Wheat Waffles because they have the potential of being contaminated with Listeria monocytogenes.No other Eggo products are implicated and there were no reports on of illness to date. Kellogg announced that the recall is a result of routine tests that the company conducts which identified the potential for contamination As soon as the company learned of a potential concern, it moved quickly to identify any foods that might be impacted and resolve the issue. Recalled product was distributed to customers and retailers in 25 states (CO, CT, DE,GA, IA, IL, IN, KS, MA, MD, ME, MI, MN, MO, ND, NE, NH, NJ,...
fasdf@sadf.tu
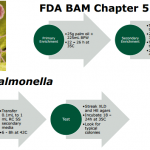
Validation of RapidCheck Select Salmonella Test Method for Detecting Low Levels of Salmonella species in Palm Oil
Objective The RapidChek SELECT Salmonella test system (RC SS) is designed to detect Salmonella species in a variety of foods and environmental surfaces, and is now also validated to detect low levels of Salmonella spp. in palm oil. The test kit permits the presumptive detection and identification of Salmonella spp. in a minimum of 22 hours when the pathogen is present at low levels in food and environmental surfaces. Challenges Method Comparison Study (FDA BAM versus RapidChek) Salmonella Typhimurium ATCC 14028 spiked at 2.5 cfu/25g sample (n=20 per method) + 5 unincculated negative controls. Cultural confirmation of all samples regardless of presumptive...